86-98-6
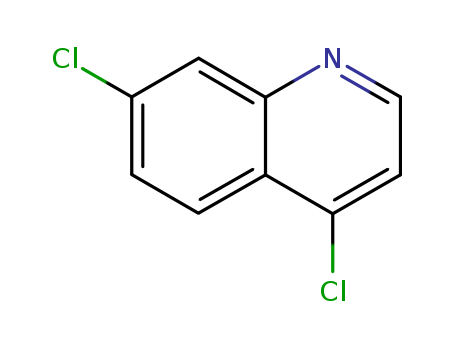
- Product Name:4,7-Dichloroquinoline
- Structural Formula:C9H5Cl2N
- Purity:99%
- Molecular Weight:198.051
Product Details
pd_meltingpoint:81-83 °C(lit.)
Appearance:white to light yellow crystal powder
High Purity 99% supply 4,7-Dichloroquinoline 86-98-6 In Bulk Supply
- Molecular Formula:C9H5Cl2N
- Molecular Weight:198.051
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.00313mmHg at 25°C
- Melting Point:81-83 °C(lit.)
- Refractive Index:1.66
- Boiling Point:292.9 °C at 760 mmHg
- PKA:1.99±0.27(Predicted)
- Flash Point:158.7 °C
- PSA:12.89000
- Density:1.407 g/cm3
- LogP:3.54160
4,7-Dichloroquinoline(Cas 86-98-6) Usage
|
Chemical Properties |
white to light yellow crystal powder |
|
Uses |
4,7-Dichloroquinoline is used in the synthesis of hybrid aminoquinoline-triazine derivatives that show anti-microbial activity. In addition, it is used in the synthesis of novel oxazolidinones as anti -microbial agents showing efficacy against common bacterial strains. Chloroquinoline impurity. |
|
General Description |
Chloroquine Related Compound A is an impurity of chloroquine, which is a 9-aminoquinoline drug that effectively inhibits viral infections in humans. |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Crystallise the dichloroquinoline from MeOH or 95% EtOH. [Beilstein 20/7 V 316.] |
InChI:InChI=1/C9H5Cl2N/c10-6-1-2-7-8(11)3-4-12-9(7)5-6/h1-5H
86-98-6 Relevant articles
Highly chemoselective deoxygenation of N-heterocyclic: N -oxides under transition metal-free conditions
Kim, Se Hyun,An, Ju Hyeon,Lee, Jun Hee
supporting information, p. 3735 - 3742 (2021/05/04)
Because their site-selective C-H functio...
Metal-Free Deoxygenation of Amine N-Oxides: Synthetic and Mechanistic Studies
Lecroq, William,Schleinitz, Jules,Billoue, Mallaury,Perfetto, Anna,Gaumont, Annie-Claude,Lalevée, Jacques,Ciofini, Ilaria,Grimaud, Laurence,Lakhdar, Sami
, p. 1237 - 1242 (2021/06/01)
We report herein an unprecedented combin...
Highly Chemoselective Deoxygenation of N-Heterocyclic N-Oxides Using Hantzsch Esters as Mild Reducing Agents
An, Ju Hyeon,Kim, Kyu Dong,Lee, Jun Hee
supporting information, p. 2876 - 2894 (2021/02/01)
Herein, we disclose a highly chemoselect...
Synthesis method of 4,7-dichloroquinoline
-
Paragraph 0020; 0027-0028; 0029; 0036-0036; 0038; 0045-0046, (2020/07/15)
The invention discloses a synthesis meth...
86-98-6 Process route
-
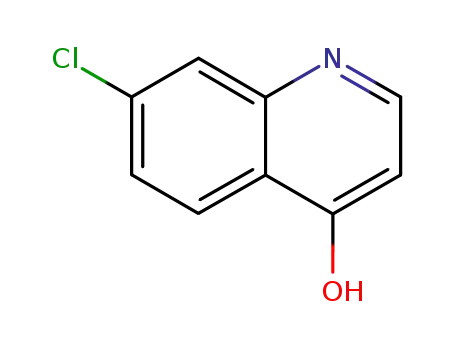
- 86-99-7
7-chloro-4-hydroxylquinoline

-
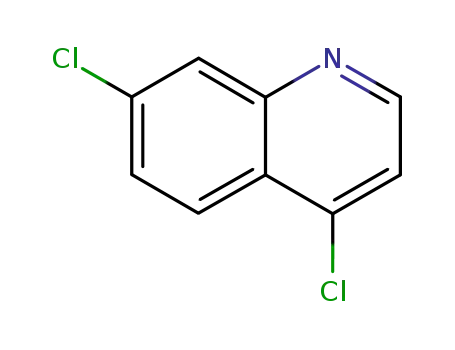
- 86-98-6,1138471-54-1
4,7-dichloroquinoline
| Conditions | Yield |
|---|---|
|
With trichlorophosphate; for 1h; Heating;
|
94% |
|
With trichlorophosphate; Reflux;
|
89.5% |
|
With trichlorophosphate; for 2h; Heating / reflux;
|
88.5% |
|
With trichlorophosphate; for 2h; Heating / reflux;
|
88.5% |
|
With trichlorophosphate; for 6h; Reflux;
|
81.6% |
|
With trichlorophosphate; for 6h; Reflux;
|
81.6% |
|
With trichlorophosphate; In toluene; at 100 - 110 ℃; for 5h;
|
77% |
|
With trichlorophosphate; for 6h; Reflux;
|
72.7% |
|
With trichlorophosphate;
|
|
|
With trichlorophosphate; at 100 ℃;
|
|
|
With trichlorophosphate; for 3h; Heating;
|
|
|
With trichlorophosphate; at 150 ℃; for 3h;
|
|
|
With bis(trichloromethyl) carbonate; N,N-dimethyl-formamide; triphenylphosphine; In toluene; at 50 - 100 ℃; Solvent; Reagent/catalyst;
|
-
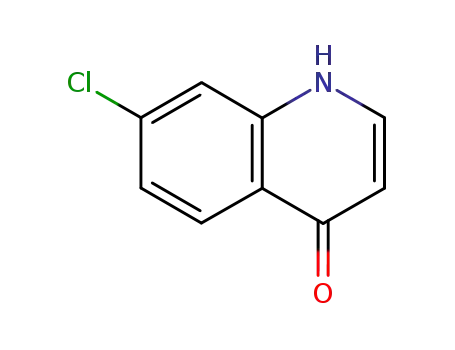
- 23833-97-8
7-chloro-4(1H)-oxoquinoline

-

- 86-98-6,1138471-54-1
4,7-dichloroquinoline
| Conditions | Yield |
|---|---|
|
With trichlorophosphate; for 2h; Reflux;
|
85% |
|
With trichlorophosphate; for 2h; Reflux;
|
85% |
|
With ((1,3,5-triazine-2,4,6-triyl)tris(oxy))tris(triphenylphosphonium) chloride; at 140 - 150 ℃; for 2h; Solvent; Ionic liquid;
|
|
|
With trichlorophosphate; Reflux;
|
86-98-6 Upstream products
-
86-99-7

7-chloro-4-hydroxylquinoline
-
25063-49-4
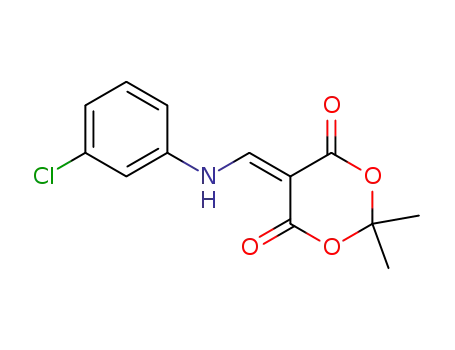
5-[(3-chloro-phenylamino)-methylene]-2,2-dimethyl-[1,3]dioxane-4,6-dione
-
39061-72-8
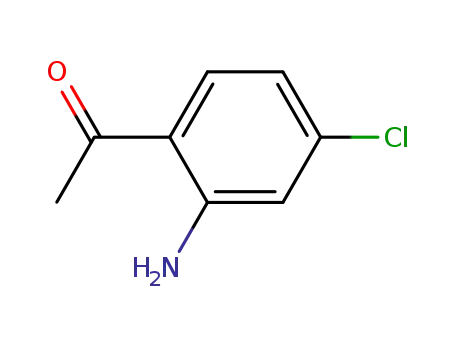
2-acetyl-5-chloroaniline
-
675578-62-8
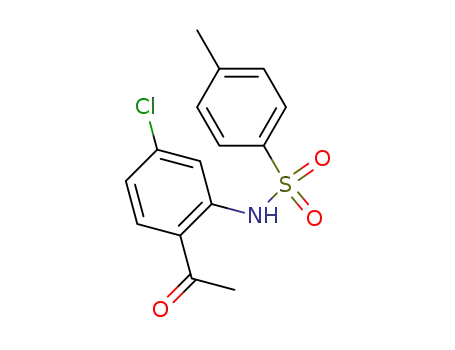
N-(2-acetyl-5-chlorophenyl)-4-methylbenzenesulfonamide
86-98-6 Downstream products
-
84594-64-9
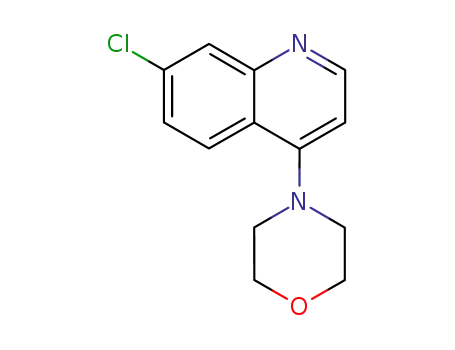
4-(7-chloroquinolin-4-yl)morpholine
-
26707-52-8
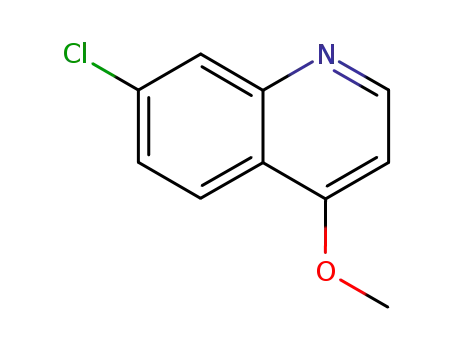
7-chloro-4-methoxyquinoline
-
24616-89-5
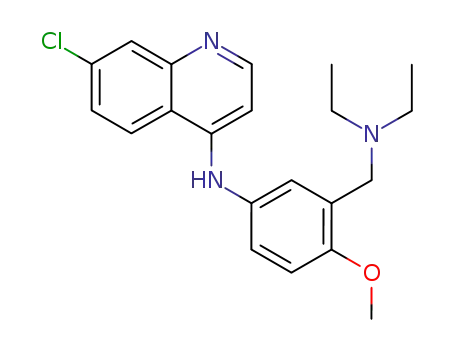
7-chloro-N-<3-(diethylamino)methyl-4-methoxyphenyl>-4-quinolamine
-
5418-53-1
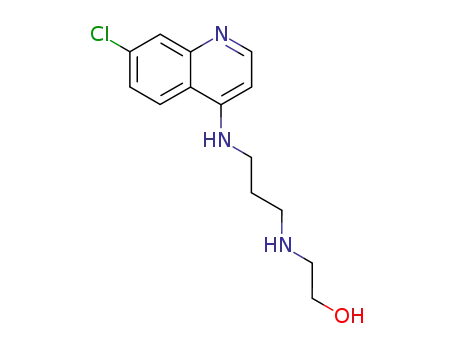
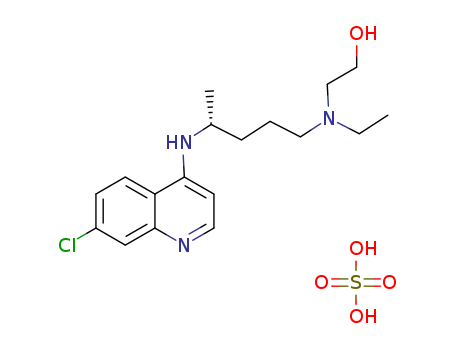
2-[3-(7-chloro-[4]quinolylamino)-propylamino]-ethanol
Relevant Products
-
Hydroxychloroquine sulfate
CAS:747-36-4