142217-78-5
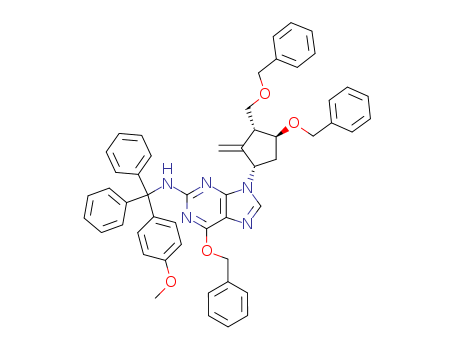
- 产品名称:(2R,3S,5S)-3-苄氧基-5-[2-[[(4-甲氧基苯基)二苯基甲基]氨基]-6-苄氧基-9H-嘌呤-9-基]-2-苄氧基甲基环戊醇
- 分子式:C52H49N5O5
- 纯度:99%
- 分子量:823.992
产品详情
外观:Colorless transparent liquid
- Molecular Formula:C52H49N5O5
- Molecular Weight:823.992
- Appearance/Colour:Colorless transparent liquid
- Refractive Index:1.645
- PKA:13.87±0.70(Predicted)
- PSA:112.78000
- Density:1.23 g/cm3
- LogP:9.61500
(2R,3S,5S)-3-(Benzyloxy)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-2-(benzyloxymethyl)cyclopentanol(Cas 142217-78-5) Usage
|
Uses |
Entecavir-?5 is used in the preparation of the anti-?HBV drug Entecavir. |
InChI:InChI=1/C52H49N5O5/c1-59-43-29-27-42(28-30-43)52(40-23-13-5-14-24-40,41-25-15-6-16-26-41)56-51-54-49-47(50(55-51)62-34-39-21-11-4-12-22-39)53-36-57(49)45-31-46(61-33-38-19-9-3-10-20-38)44(48(45)58)35-60-32-37-17-7-2-8-18-37/h2-30,36,44-46,48,58H,31-35H2,1H3,(H,54,55,56)/t44-,45-,46-,48?/m0/s1
142217-78-5 Relevant articles
Improved entecavir intermediate synthesis process and improved entecavir synthesis process
-
Paragraph 0012; 0042-0047; 0051; 0053; 0055; 0057; 0058, (2020/10/14)
The invention discloses an improved ente...
BMS-200475, a novel carbocyclic 2'-deoxyguanosine analog with potent and selective anti-hepatitis B virus activity in vitro
Bisacchi,Chao,Bachard,Daris,Innaimo,Jacobs,Kocy,Lapointe,Martel,Merchant,Slusarchyk,Sundeen,Young,Colonno,Zahler
, p. 127 - 132 (2007/10/03)
BMS-200475, a never carbocyclic analog o...
Hydroxymethyl (methylenecyclopentyl) purines and pyrimidines
-
, (2008/06/13)
Antiviral activity is exhibited by compo...
142217-78-5 Process route
-
![[1S-(1α,2β,3α,5β)]-5-(2-amino-6-benzyloxy-9H-purin-9-yl)-3-benzyloxy-2-(benzyloxy)methyl-cyclopentanol](/upload/2023/9/ab4dbbed-38ea-4054-9e98-7f23a5d1b227.png)
- 142217-77-4
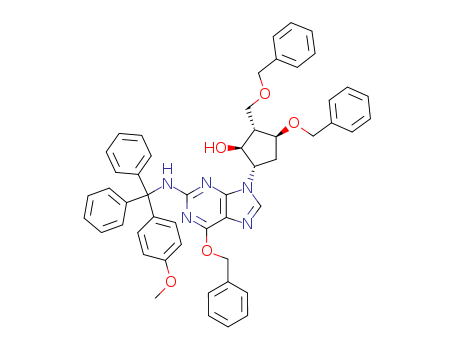
[1S-(1α,2β,3α,5β)]-5-(2-amino-6-benzyloxy-9H-purin-9-yl)-3-benzyloxy-2-(benzyloxy)methyl-cyclopentanol

-
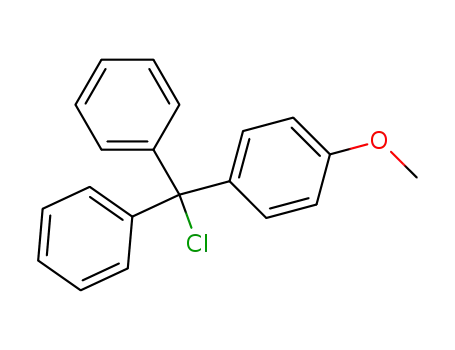
- 14470-28-1
mono-4-methoxytrityl chloride

-
![(1S,2S,3S,5S)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-benzyloxy-9H-purin-9-yl]-3-benzyloxy-2-[(benzyloxy)methyl]cyclopentanol](/upload/2023/9/36b6bb5b-ea07-45ae-9109-5cac4035404f.png)
- 142217-78-5
(1S,2S,3S,5S)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-benzyloxy-9H-purin-9-yl]-3-benzyloxy-2-[(benzyloxy)methyl]cyclopentanol
| Conditions | Yield |
|---|---|
|
With dmap; TEA; In dichloromethane;
|
82% |
|
With dmap; triethylamine; In dichloromethane; at 20 - 30 ℃; for 1h; Concentration; Inert atmosphere;
|
-
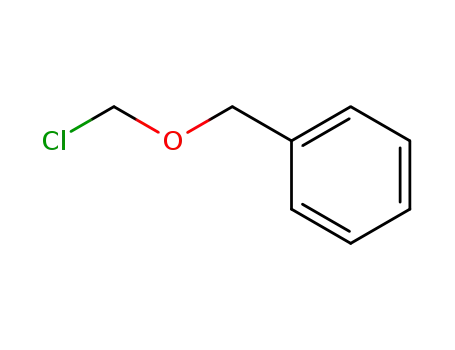
- 3587-60-8
Benzyloxymethyl chloride

-
![(1S,2S,3S,5S)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-benzyloxy-9H-purin-9-yl]-3-benzyloxy-2-[(benzyloxy)methyl]cyclopentanol](/upload/2023/9/36b6bb5b-ea07-45ae-9109-5cac4035404f.png)
- 142217-78-5
(1S,2S,3S,5S)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-benzyloxy-9H-purin-9-yl]-3-benzyloxy-2-[(benzyloxy)methyl]cyclopentanol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 6 steps
1: tetrahydrofuran / -78 - -65 °C
2: 1.) diisopinylcampheylborane (prepared from (+)-α-pinene), 2.) aq. NaOH, H2O2 / 1.) THF, -65 to -78 deg C
3: VO(acac)2, t-BuOOH / CH2Cl2
4: NaH, Bu4NI / dimethylformamide
5: 60 percent / LiH / dimethylformamide / 2 h / 125 °C
6: 82 percent / TEA, DMAP / CH2Cl2
With tert.-butylhydroperoxide; dmap; sodium hydroxide; bis(acetylacetonate)oxovanadium; (-)-diisopinocampheylborane; TEA; dihydrogen peroxide; tetra-(n-butyl)ammonium iodide; lithium hydride; sodium hydride; In tetrahydrofuran; dichloromethane; N,N-dimethyl-formamide;
|
142217-78-5 Upstream products
-
142217-77-4
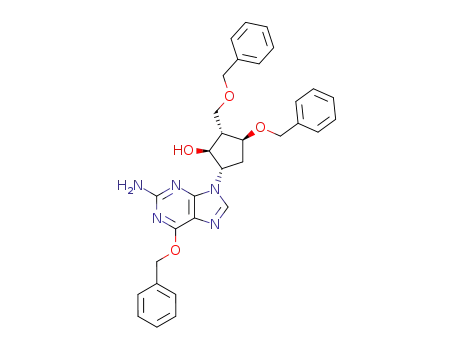
[1S-(1α,2β,3α,5β)]-5-(2-amino-6-benzyloxy-9H-purin-9-yl)-3-benzyloxy-2-(benzyloxy)methyl-cyclopentanol
-
14470-28-1

mono-4-methoxytrityl chloride
-
3587-60-8

Benzyloxymethyl chloride
-
39939-07-6
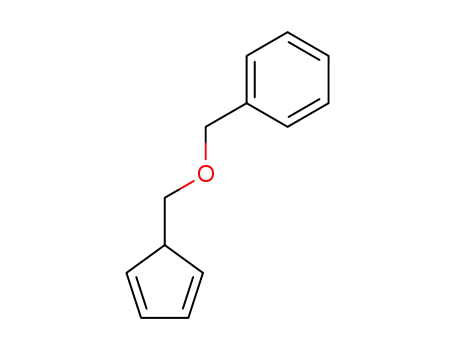
5-(benzyloxymethyl)cyclopentadiene
142217-78-5 Downstream products
-
142217-79-6
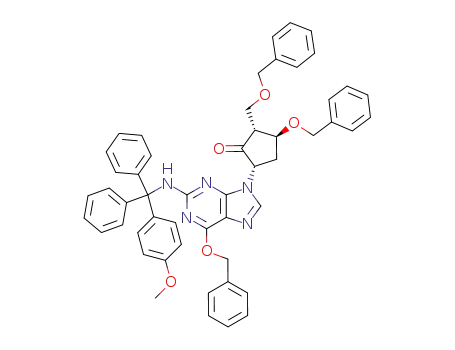
(2R,3S,5S)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-benzyloxy-9H-fluoren-9-yl]-3-benzyloxy-2-[(benzyloxy)methyl]cyclopentanone
-
142217-80-9
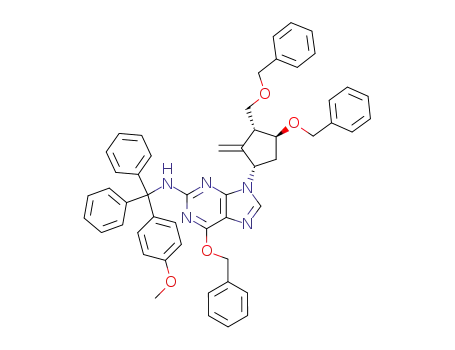
6-(benzyloxy)-9-((1S,3R,4S)-4-(benzyloxy)-3-((benzyloxy)methyl)-2-methylidenecyclopentyl)-N-((4-methoxyphenyl)diphenylmethyl)-9H-purin-2-amine
-
142217-69-4
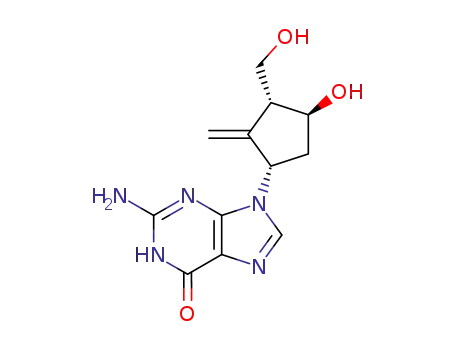
entecavir
-
142217-81-0
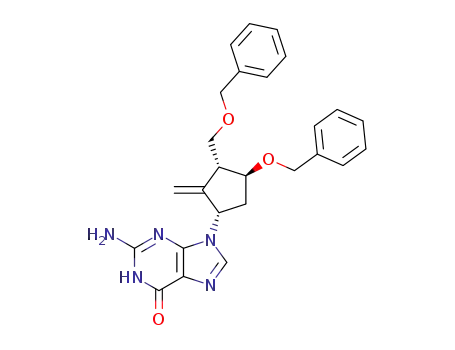
2-amino-1,9-dihydro-9-[(1S,3R,4S)-2-methylene-4-(phenylmethoxy)-3-[(phenylmethoxy)methyl]cyclopentyl]-6H-purin-6-one
相关产品
-
(2-(甲基氨基)吡啶-3-基)甲基 2-((叔丁氧羰基)(甲基)氨基)乙酸酯
CAS:1180002-01-0