产品详情
- Molecular Formula:C20H21N3O4
- Molecular Weight:367.404
- PSA:86.67000
- LogP:3.04396
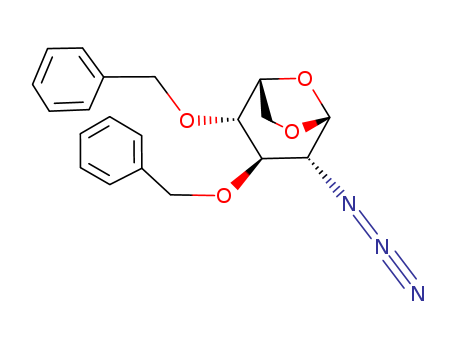
1,6-Anhydro-2-azido-2-deoxy-3,4-bis-O-(phenylmethyl)-beta-D-glucopyranose(Cas 55682-48-9) Usage
InChI:InChI=1/C20H21N3O4/c21-23-22-17-19(25-12-15-9-5-2-6-10-15)18(16-13-26-20(17)27-16)24-11-14-7-3-1-4-8-14/h1-10,16-20H,11-13H2/t16-,17-,18-,19-,20-/m1/s1
55682-48-9 Relevant articles
Oligosaccharide compound and its manufacture and its intermediate
-
Paragraph 0242; 0243; 0247, (2018/04/14)
The purpose of the present invention is ...
Preparation method of fondaparinux sodium monosaccharide intermediate
-
Paragraph 0068; 0091; 0099; 0099, (2019/01/16)
The invention discloses a preparation me...
Synthesis and in vitro cytotoxicity of acetylated 3-fluoro, 4-fluoro and 3,4-difluoro analogs of D-glucosamine and D-galactosamine
Horník, ?těpán,?t'astná, Lucie ?ervenková,Cu?ínová, Petra,Sykora, Jan,Káňová, Kate?ina,Hrstka, Roman,Císa?ová, Ivana,Dra?ínsky, Martin,Karban, Jind?ich
supporting information, p. 750 - 759 (2016/07/06)
Background: Derivatives of D-glucosamine...
Total synthesis of anticoagulant pentasaccharide fondaparinux
Li, Tiehai,Ye, Hui,Cao, Xuefeng,Wang, Jiajia,Liu, Yonghui,Zhou, Lifei,Liu, Qiang,Wang, Wenjun,Shen, Jie,Zhao, Wei,Wang, Peng
, p. 1071 - 1080 (2014/05/20)
The anticoagulant pentasaccharide fondap...
55682-48-9 Process route
-
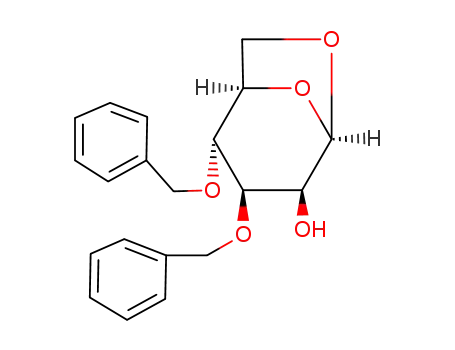
- 67227-89-8
1,6-anhydro-3,4-di-O-benzyl-β-D-mannopyranoside

-
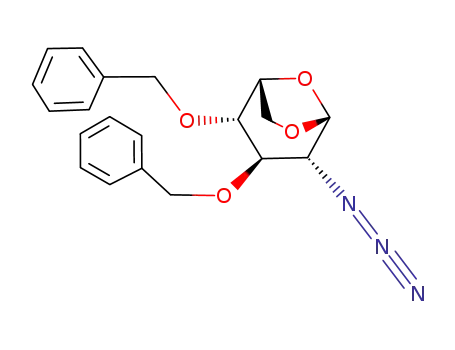
- 55682-48-9
1,6-anhydro-2-azido-3,4-di-O-benzyl-2-deoxy-D-glucopyranose
| Conditions | Yield |
|---|---|
|
1,6-anhydro-3,4-di-O-benzyl-β-D-mannopyranoside; With pyridine; trifluoromethylsulfonic anhydride; In dichloromethane; at -10 - 0 ℃; for 1h; Inert atmosphere;
With sodium azide; In N,N-dimethyl-formamide; at 20 ℃; for 2h; Molecular sieve;
|
85.1% |
|
Multi-step reaction with 2 steps
1: pyridine / CH2Cl2
2: LiN3 / dimethylformamide / Ambient temperature
With pyridine; lithium azide; In dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: pyridine / CH2Cl2 / 0.83 h / -10 - 0 °C
2: NaN3 / dimethylformamide / 0.25 h
With pyridine; sodium azide; In dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: pyridine / CH2Cl2 / 2 h / 0 °C
2: NaN3, molecular sieves (4A) / dimethylformamide / Ambient temperature
With pyridine; sodium azide; 4 A molecular sieve; In dichloromethane; N,N-dimethyl-formamide;
|
-
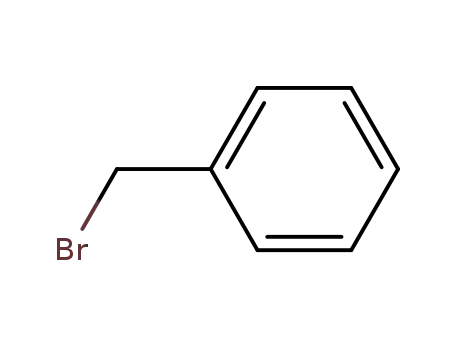
- 100-39-0
benzyl bromide

-
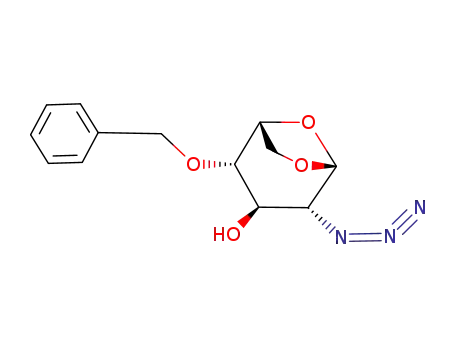
- 55682-47-8
1,6-anhydro-2-azido-4-O-benzyl-2-deoxy-β-D-glucopyranose

-

- 55682-48-9
1,6-anhydro-2-azido-3,4-di-O-benzyl-2-deoxy-D-glucopyranose
| Conditions | Yield |
|---|---|
|
With sodium hydride; In N,N-dimethyl-formamide; for 1h;
|
100% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 3h;
|
95% |
|
With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 2.08333h; Inert atmosphere;
|
55682-48-9 Upstream products
-
100-39-0
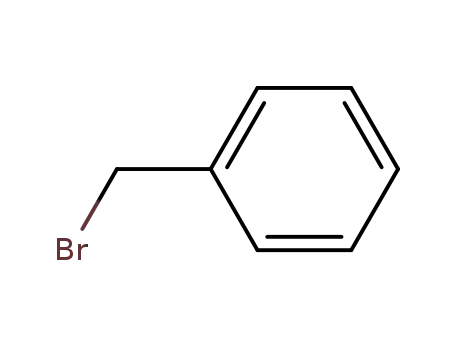
benzyl bromide
-
67546-20-7
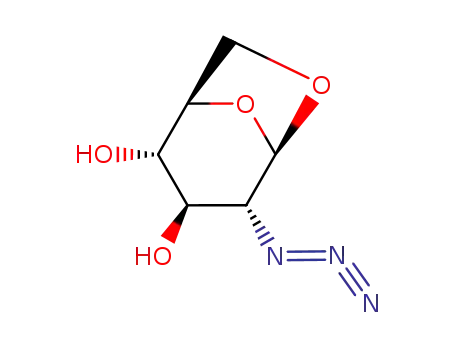
1,6-anhydro-2-azido-2-deoxy-β-D-glucopyranose
-
55682-47-8

1,6-anhydro-2-azido-4-O-benzyl-2-deoxy-β-D-glucopyranose
-
97292-02-9
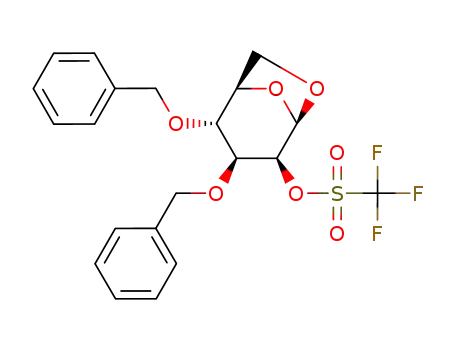
1,6-anhydro-3,4-di-O-benzyl-2-O-<(trifluoromethyl)sulfonyl>-β-D-mannopyranose
55682-48-9 Downstream products
-
214756-90-8
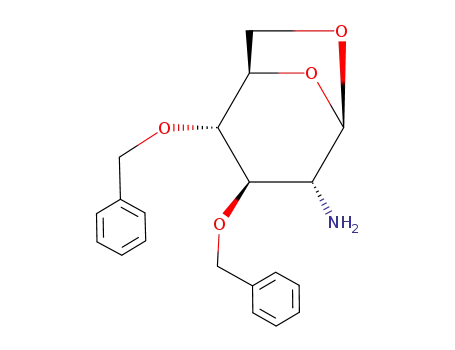
1,6-anhydro-2-amino-2-deoxy-3,4-di-O-benzyl-β-D-glucopyranose
-
55682-57-0
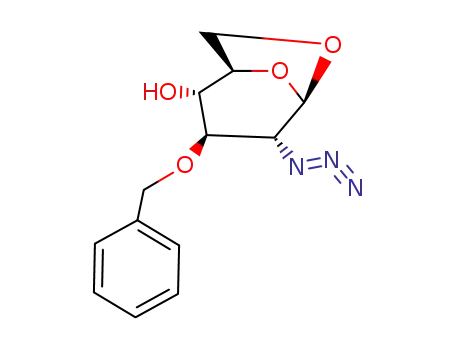
1,6-anhydro-2-azido-3-O-benzyl-2-deoxy-β-D-glucopyranose
-
372494-01-4
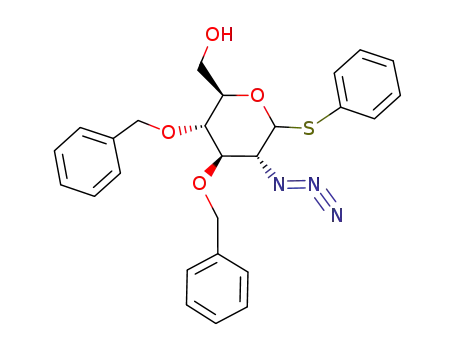
((2R,3S,4R,5R)-5-Azido-3,4-bis-benzyloxy-6-phenylsulfanyl-tetrahydro-pyran-2-yl)-methanol
-
50447-93-3
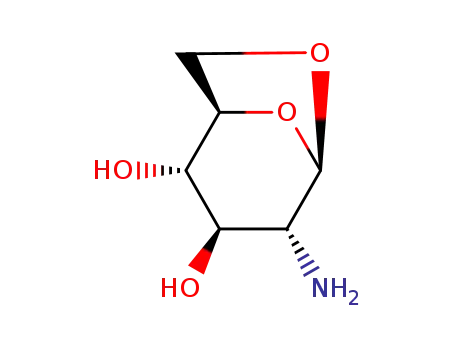
2-amino-1,6-anhydro-2-deoxy-β-D-glucopyranose
相关产品
-
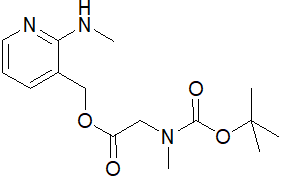
(2-(甲基氨基)吡啶-3-基)甲基 2-((叔丁氧羰基)(甲基)氨基)乙酸酯
CAS:1180002-01-0
-
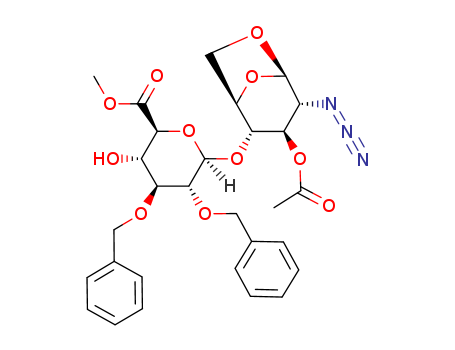
磺达肝葵钠中间体EF二糖
CAS:99541-26-1
-
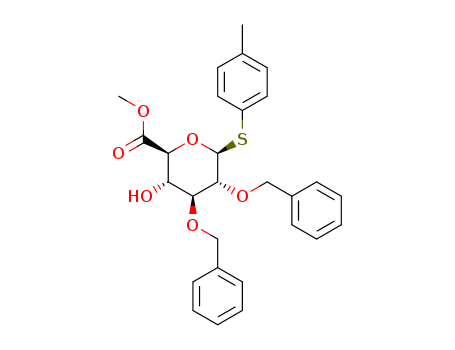
4-甲基苯基 2,3-二-O-苄基-1-硫代-BETA-D-吡喃葡糖苷酸甲酯
CAS:1352561-81-9