32399-12-5
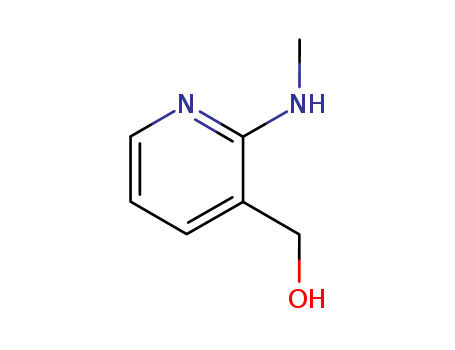
- Product Name:2-(Methylamino)pyridine-3-methanol
- Structural Formula:C7H10N2O
- Purity:99%
- Molecular Weight:138.169
Product Details
Factory supply Pharmaceutical Intermediates 2-(Methylamino)pyridine-3-methanol 32399-12-5 manufacturer
- Molecular Formula:C7H10N2O
- Molecular Weight:138.169
- Vapor Pressure:0.00023mmHg at 25°C
- Refractive Index:1.613
- Boiling Point:312.2 °C at 760 mmHg
- PKA:13.63±0.10(Predicted)
- Flash Point:142.6 °C
- PSA:45.15000
- Density:1.191 g/cm3
- LogP:0.68860
2-(Methylamino)pyridine-3-methanol(Cas 32399-12-5) Usage
InChI:InChI=1/C7H10N2O/c1-8-7-6(5-10)3-2-4-9-7/h2-4,10H,5H2,1H3,(H,8,9)
32399-12-5 Relevant articles
-
Blanch,Fretheim
, p. 1892 (1971)
-
A pharmaceutical intermediate 2 - methylamino -3 - pyridine methanol preparation method (by machine translation)
-
Paragraph 0067-0074, (2019/10/22)
The invention relates to a shakang [...]...
INHIBITORS OF INDOLEAMINE 2,3-DIOXYGENASE AND METHODS OF THEIR USE
-
Page/Page column 100; 101; 102, (2019/07/20)
The present invention provides a compoun...
A preparation method of the midbody shakang zuo
-
Paragraph 0039-0046, (2018/05/16)
The invention relates to a preparation m...
RING-FUSED 2-PYRIDONE DERIVATIVES AND HERBICIDES
-
Page/Page column 69, (2011/12/12)
Provided are 2-pyridone derivatives whic...
32399-12-5 Process route
-
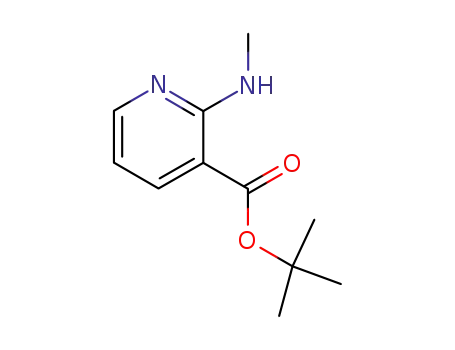
-
338990-70-8
tert-butyl 2-(methylamino)pyridine-3-carboxylate

-
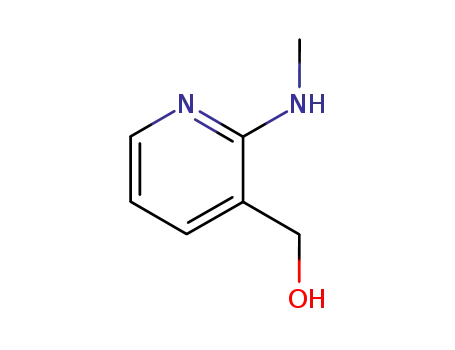
-
32399-12-5
2-(N-methylamino)-3-hydroxymethylpyridine
| Conditions | Yield |
|---|---|
|
With
potassium borohydride; zinc(II) chloride;
In
tetrahydrofuran; toluene;
Reagent/catalyst;
Solvent;
Reflux;
|
88.2% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 ℃;
|
80% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 3.5h;
|
76% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 2h;
|
74.6% |
-
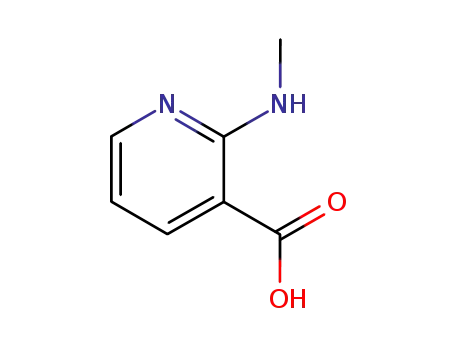
-
32399-13-6
2-(methylamino)pyridine-3-carboxylic acid

-

-
32399-12-5
2-(N-methylamino)-3-hydroxymethylpyridine
| Conditions | Yield |
|---|---|
|
With
sodium bis(2-methoxyethoxy)aluminium dihydride;
In
toluene;
at 20 - 60 ℃;
Reagent/catalyst;
Temperature;
Solvent;
|
88.1% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
for 24h;
Heating;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 20 ℃;
Inert atmosphere;
Cooling with ice;
|
32399-12-5 Upstream products
-
32399-13-6

2-(methylamino)pyridine-3-carboxylic acid
-
338990-70-8

tert-butyl 2-(methylamino)pyridine-3-carboxylate
-
49609-84-9
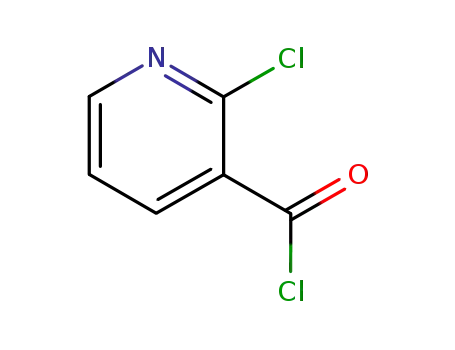
2-Chloronicotinoyl chloride
-
2942-59-8
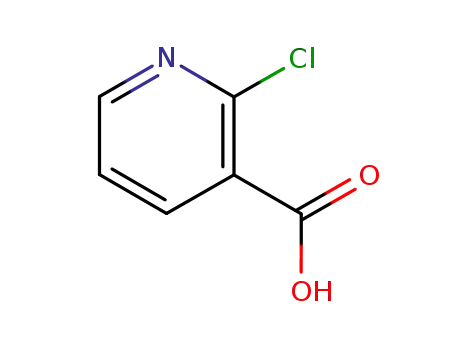
2-chloronicotinic acid
32399-12-5 Downstream products
-
338990-31-1
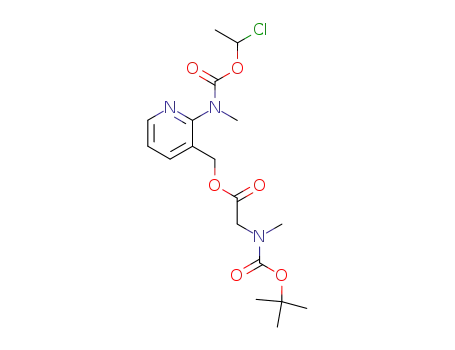
N-methyl N-(3-[((N-tert-butoxycarbonyl-N-methylamino)acetoxy)methyl]pyridin-2-yl)carbamic acid (1-chloroethyl) ester
-
338990-63-9
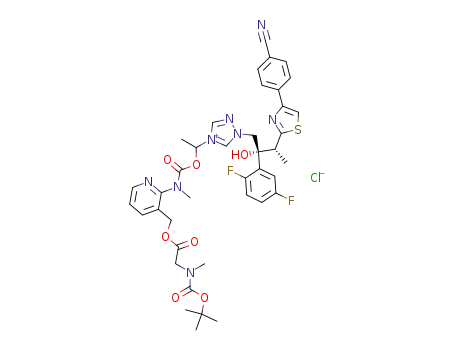
1-[[N-methyl-N-3-[(t-butoxycarbonylmethylamino)acetoxymethyl]pyridin-2-yl]carbamoyloxy]ethyl-1-[(2R,3R)-2-(2,5-difluorophenyl)-2-hydroxy-3-[4-(4-cyanophenyl)thiazol-2-yl]butyl]-1H-[1,2,4]triazol-4-ium chloride
-
128271-33-0
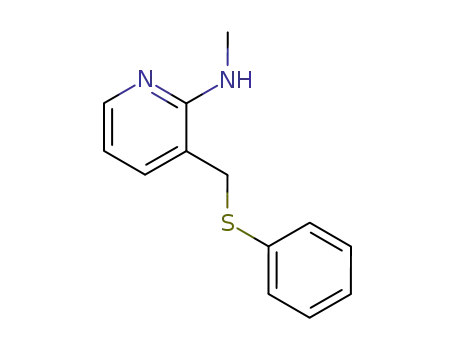
Methyl-(3-phenylsulfanylmethyl-pyridin-2-yl)-amine
-
127555-45-7
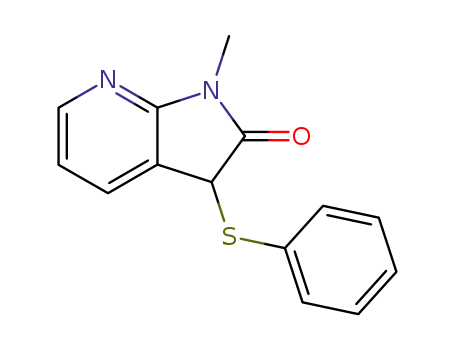
1-Methyl-3-phenylsulfanyl-1,3-dihydro-pyrrolo[2,3-b]pyridin-2-one
Relevant Products
-
6-O-Benzylguanine
CAS:19916-73-5