110567-21-0
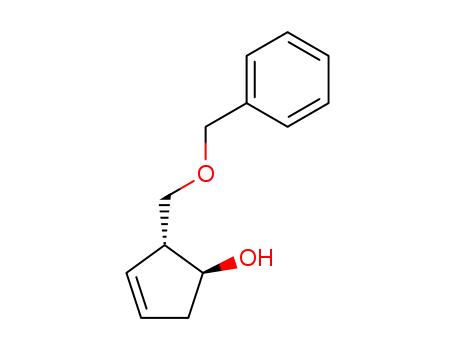
- Product Name:(1S, 2R)-2-(Benzyloxymethyl)-1-hydroxy-3-cyclopentene
- Structural Formula:C13H16O2
- Purity:99%
- Molecular Weight:204.269
Product Details
Factory Supply commercial production 110567-21-0, (1S, 2R)-2-(Benzyloxymethyl)-1-hydroxy-3-cyclopentene manufacturer
- Molecular Formula:C13H16O2
- Molecular Weight:204.269
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.565
- Boiling Point:318.906 °C at 760 mmHg
- PKA:14.65±0.40(Predicted)
- Flash Point:135.492 °C
- PSA:29.46000
- Density:1.112 g/cm3
- LogP:2.14020
(1S, 2R)-2-(Benzyloxymethyl)-1-hydroxy-3-cyclopentene(Cas 110567-21-0) Usage
|
Uses |
(1S,2R)-2-((Benzyloxy)methyl)cyclopent-3-enol is an intermediate in the synthesis of Entecavir (E558900), an oral antiviral drug used in the treatment of hepatitis B infection. |
InChI:InChI=1/C13H16O2/c14-13-8-4-7-12(13)10-15-9-11-5-2-1-3-6-11/h1-7,12-14H,8-10H2/t12-,13+/m1/s1
110567-21-0 Relevant articles
PCSK9 INHIBITORS AND METHODS OF USE THEREOF
-
Page/Page column 146-147, (2020/07/31)
The invention relates to a novel inhibit...
STING MODULATOR COMPOUNDS, AND METHODS OF MAKING AND USING
-
Paragraph 0216, (2019/05/30)
The present disclosure provides STING mo...
Preparation method of optical pure cyclopentene alcohol serving as medical intermediate
-
Paragraph 0041; 0042, (2017/10/09)
The invention relates to a preparation m...
110567-21-0 Process route
-
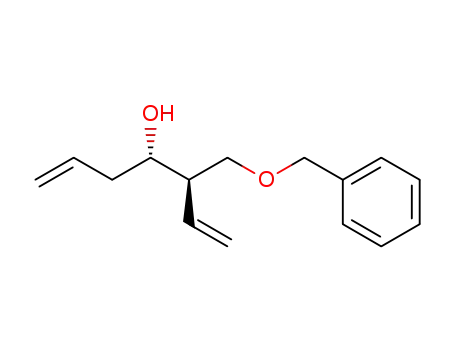
-
(3R,4S)-3-benzyloxymethylhepta-1,6-dien-4-ol

-
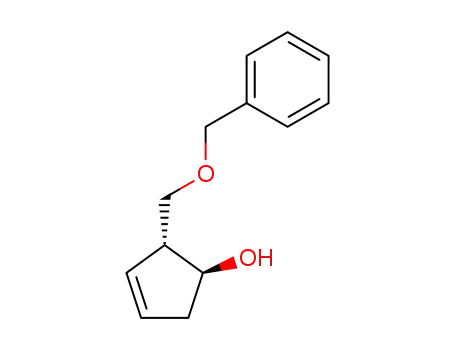
- 110567-21-0
(1S,2R)-2-(benzyloxymethyl)-1-hydroxy-3-cyclopentene
| Conditions | Yield |
|---|---|
|
With modified Grubb's catalyst; SBA-15; In cyclohexane; at 25 ℃; for 3h;
|
82% |
-
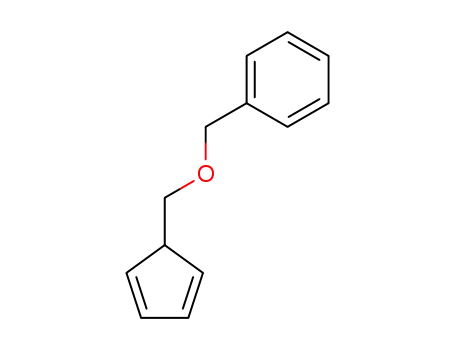
- 39939-07-6
5-(benzyloxymethyl)cyclopentadiene

-

- 110567-21-0
(1S,2R)-2-(benzyloxymethyl)-1-hydroxy-3-cyclopentene
| Conditions | Yield |
|---|---|
|
With (-)-diisopinan-3-ylborane; In tetrahydrofuran; 1.) -60 deg C, 1 h; 2.) 5 deg C, 18 h;
|
15.8 g |
|
With sodium hydroxide; (-)-diisopinocampheylborane; dihydrogen peroxide; Yield given. Multistep reaction; 1.) THF, -65 to -78 deg C;
|
|
|
5-(benzyloxymethyl)cyclopentadiene; With diisopinocampheylborane; In tetrahydrofuran; at -60 - 0 ℃; for 17h;
With sodium hydroxide; dihydrogen peroxide; In tetrahydrofuran; diethyl ether; at 0 ℃; for 12h;
|
8.20 g |
|
5-(benzyloxymethyl)cyclopentadiene; With diisopropylcamphenylborane;
With dihydrogen peroxide; Further stages.;
|
|
|
5-(benzyloxymethyl)cyclopentadiene; With (-)-IPC2BH; In tetrahydrofuran; at -78 - -20 ℃; for 48h;
With dihydrogen peroxide; sodium hydroxide; In water; at 20 ℃; for 12h;
|
|
|
5-(benzyloxymethyl)cyclopentadiene; With diisopinocampheylborane; In tetrahydrofuran; at -78 - -10 ℃; Inert atmosphere;
With dihydrogen peroxide; sodium hydroxide; In methanol; water; at 20 ℃; for 24h; Inert atmosphere;
|
5.04 g |
|
5-(benzyloxymethyl)cyclopentadiene; With diisopinocampheylborane; In tetrahydrofuran; at -78 - -10 ℃; for 72h; Inert atmosphere;
With dihydrogen peroxide; sodium hydroxide; In tetrahydrofuran; methanol; at 20 ℃; for 24h; Inert atmosphere;
|
5.04 g |
|
5-(benzyloxymethyl)cyclopentadiene; In tetrahydrofuran; at -78 - 0 ℃; for 72h; Inert atmosphere;
With dihydrogen peroxide; sodium hydroxide; In methanol; water; at 20 ℃; for 24h;
|
4.8 g |
|
5-(benzyloxymethyl)cyclopentadiene; With diisopinocampheylborane; In tetrahydrofuran; at -78 - -10 ℃; for 72h;
With dihydrogen peroxide; sodium hydroxide; In water; at 20 ℃; for 24h;
|
4.8 g |
110567-21-0 Upstream products
-
39939-07-6

5-(benzyloxymethyl)cyclopentadiene
-
3587-60-8
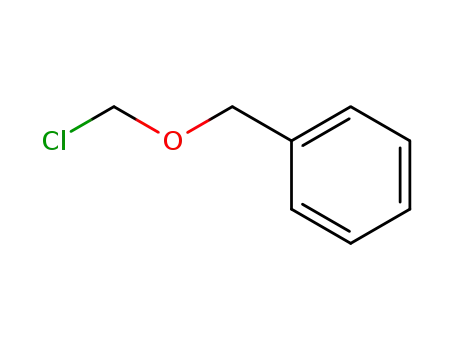
Benzyloxymethyl chloride
-
4984-82-1
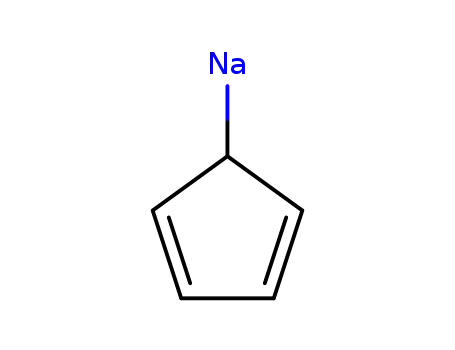
sodium cyclopentadienylide
-
668996-17-6
(1R,4S,5S)-5-((benzyloxy)methyl)-4-((4-methoxybenzyl)oxy)cyclopent-2-enol
110567-21-0 Downstream products
-
191480-67-8
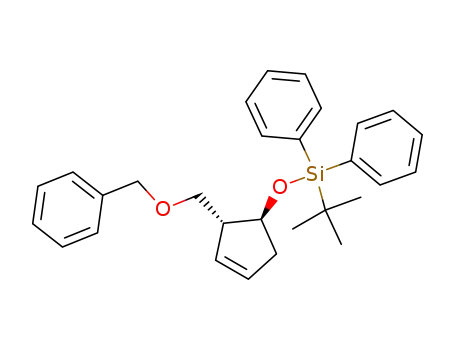
((1S,2R)-2-Benzyloxymethyl-cyclopent-3-enyloxy)-tert-butyl-diphenyl-silane
-
325480-40-8
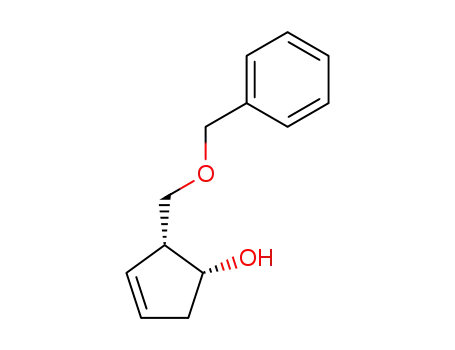
(1R,2R)-2-benzyloxymethyl-3-cyclopenten-1-ol
-
325480-41-9
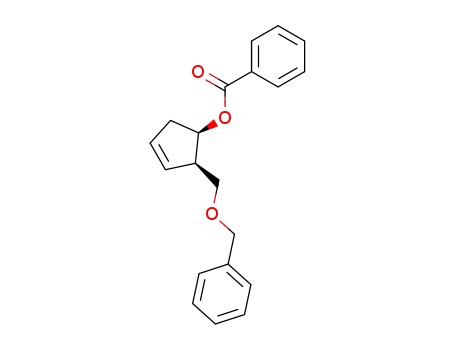
(1R,2R)-2-benzyloxymethyl-3-cyclopenten-1-yl benzoate
-
325464-21-9
(1R,2R,3S,5R)-2-[(phenylmethoxy)methyl]bicyclo[3.1.0]hexan-3-ol
Relevant Products
-
Isavuconazonium sulfate
CAS:946075-13-4