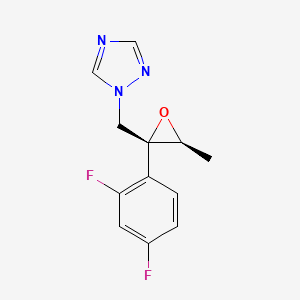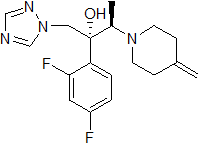
164650-44-6
- Product Name:Efinaconazole
- Structural Formula:C18H22F2N4O
- Purity:99%
- Molecular Weight:348.39904
Product Details
- Molecular Formula:C18H22F2N4O
- Molecular Weight:348.39904
- Boiling Point:512.2±60.0 °C(Predicted)
- PKA:12.11±0.29(Predicted)
- PSA:54.18000
- Density:1.26±0.1 g/cm3(Predicted)
- LogP:2.42250
164650-44-6 Relevant articles
Identification, synthesis, and control of efinaconazole impurities
Zhu, Fuqiang,Zhang, Jian,Xiamuxi, Hainimu,Chen, Weiming,Hu, Tianwen,Yang, Xiaojun,Tian, Guanghui,Ni, Runyan,Li, Jian,Suo, Jin,Xie, Yuanchao,Shen, Jingshan,Aisa, Haji A.,He, Yang
, p. 438 - 441 (2018)
Impurities A-F were observed, identified...
A Facile Epoxide Aminolysis Promoted by (t-BuO)2Mg and Its Application to the Synthesis of Efinaconazole
Zhu, Fuqiang,Xie, Yuanchao,Zhang, Jian,Tian, Guanghui,Qin, Hongjian,Yang, Xiaojun,Hu, Tianwen,He, Yang,Aisa, Haji A.,Shen, Jingshan
, p. 625 - 632 (2018)
A novel and efficient method for the ami...
Asymmetric Catalytic Epoxidation of Terminal Enones for the Synthesis of Triazole Antifungal Agents
Feng, Xiaoming,He, Qianwen,Liu, Xiaohua,Zhang, Dong,Zhang, Fengcai
supporting information, p. 6961 - 6966 (2021/09/11)
An enantioselective epoxidation of α-sub...
PREPARATION METHOD FOR EFINACONAZOLE
-
Paragraph 0031-0048, (2021/11/26)
The present invention provides a prepara...
NOVEL METHOD FOR PREPARATION OF EPOXYTRIAZOLE DERIVATIVES
-
Paragraph 0109-0110; 0119-0121, (2021/04/13)
The present invention relates to novel p...
METHOD FOR PREPARATION OF EFINACONAZOLE IN IONIC LIQUID MEDIUM
-
Paragraph 0079; 0095-0102, (2021/01/29)
The present invention relates to a novel...
164650-44-6 Process route
-
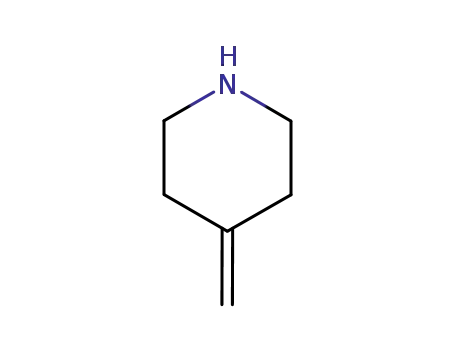
- 148133-82-8
4-methylene piperidine

-
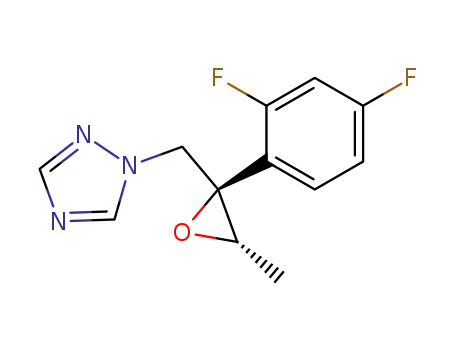
- 124627-86-7,132563-70-3,132563-77-0,135270-07-4,135270-10-9,135270-13-2,141611-70-3,127000-90-2
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole

-
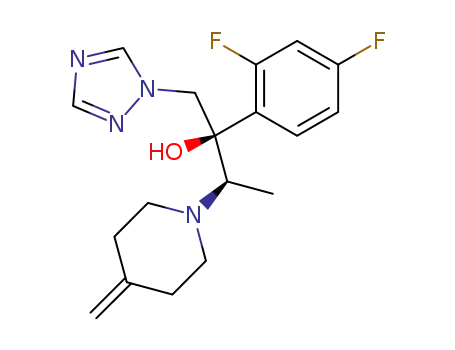
- 164650-45-7,164650-44-6
(2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidine-1-yl)-1-(1H-1,2,4-triazole-1-yl)-butane-2-ol
| Conditions | Yield |
|---|---|
|
With strontium perchlorate hydrate; In acetonitrile; at 90 ℃; for 1.5h; Reagent/catalyst; Solvent; Temperature;
|
98% |
|
In ethanol; at 120 ℃; for 6h; enantioselective reaction; Inert atmosphere; Microwave irradiation;
|
90% |
|
In ethanol; at 120 ℃; for 6h; Microwave irradiation;
|
84% |
|
4-methylene piperidine; With lithium hydroxide; In acetonitrile; for 0.25h;
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; With potassium iodide; In acetonitrile; Reflux;
|
75.8% |
|
4-methylene piperidine; 1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; With lithium bromide; In acetonitrile;
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; In acetonitrile; for 24h; Reflux;
|
72.46% |
|
In ethanol; water; at 85 ℃; for 24h;
|
54% |
|
With zinc(II) chloride; In tert-Amyl alcohol; for 24h; Reagent/catalyst; Reflux;
|
1 g |
-

- 124627-86-7,132563-70-3,132563-77-0,135270-07-4,135270-10-9,135270-13-2,141611-70-3,127000-90-2
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole

-
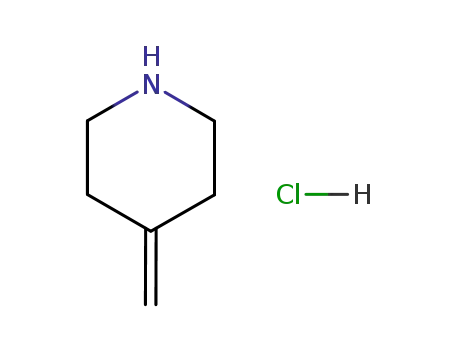
- 144230-50-2
4-methylenepiperidine monohydrochloride

-

- 164650-45-7,164650-44-6
(2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidine-1-yl)-1-(1H-1,2,4-triazole-1-yl)-butane-2-ol
| Conditions | Yield |
|---|---|
|
4-methylenepiperidine monohydrochloride; With sodium hydroxide; In acetonitrile; at 25 ℃; for 0.5h;
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; With lithium iodide; In acetonitrile; at 85 ℃; for 5h; Reagent/catalyst;
|
96% |
|
With potassium iodide; lithium hydroxide; In acetonitrile; for 8h; Reagent/catalyst; Solvent; Reflux;
|
87.8% |
|
With potassium hydroxide; lithium bromide; In acetonitrile; at 85 - 90 ℃; for 20h; Reagent/catalyst; Solvent; Large scale;
|
86.91% |
|
With N-ethyl-N,N-diisopropylamine; magnesium chloride; In acetonitrile; at 0 - 75 ℃; for 16h; Reagent/catalyst; Temperature; Solvent;
|
84% |
|
4-methylenepiperidine monohydrochloride; With potassium hydroxide; In water; at 23 - 28 ℃; for 2h;
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; With 1-ethyl-3-methylimidazol-3-ium ethyl sulfate; at 100 ℃; for 6h; Temperature;
|
80% |
|
4-methylenepiperidine monohydrochloride; With potassium hydroxide; In water; at 23 - 28 ℃; for 2h;
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; With water; 1-ethyl-3-methylimidazol-3-ium ethyl sulfate; at 100 ℃; for 6h;
|
80% |
|
With potassium hydroxide; sodium chloride; In ethanol; hexane; ethyl acetate;
|
|
|
4-methylenepiperidine monohydrochloride; With water; lithium carbonate; lithium bromide; In ethanol; at 0.25 - 30 ℃; for 72h; Reflux;
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; In ethanol; for 72h; Solvent; Reagent/catalyst; Temperature; Reflux;
|
|
|
4-methylenepiperidine monohydrochloride; With sodium hydroxide; In dichloromethane; at 0 - 5 ℃; for 1h;
1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methyloxiran-2-yl)-methyl)-1H-1,2,4-triazole; With lithium bromide; In acetonitrile; at 20 - 100 ℃; Solvent;
|
164650-44-6 Upstream products
-
148133-82-8

4-methylene piperidine
-
135270-07-4
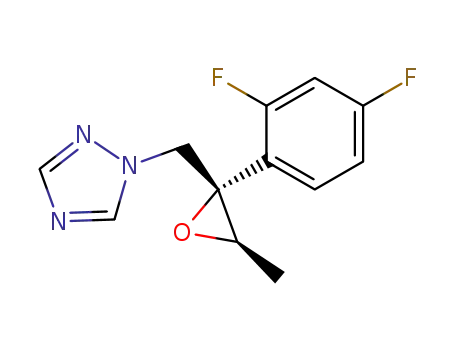
(2S,3R)-2-(2,4-difluorophenyl)-3-methyl-2-<(1H-1,2,4-triazol-1-yl)methyl>oxirane
-
63608-15-1
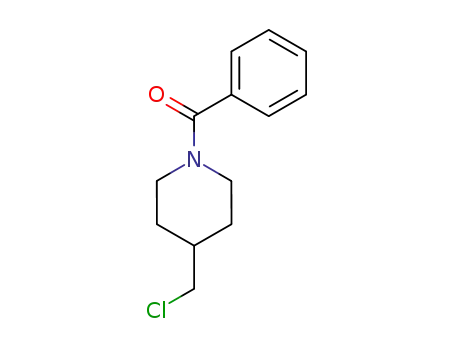
(4-(chloromethyl)piperidin-1-yl)(phenyl)methanone
-
188904-84-9
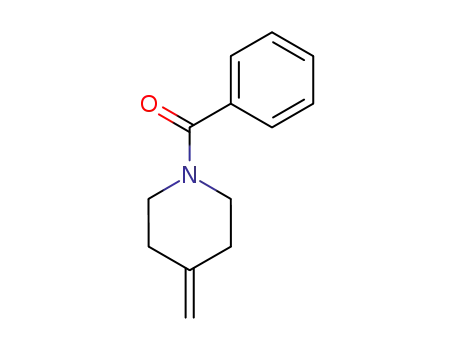
N-benzoyl-4-methylenepiperidine