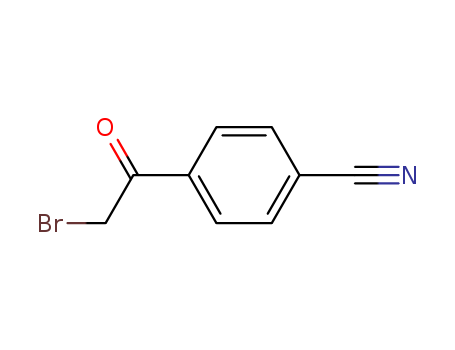
产品详情
pd_meltingpoint:92-96 °C(lit.)
外观:off-white to light yellow crystalline powder
- Molecular Formula:C9H6BrNO
- Molecular Weight:224.057
- Appearance/Colour:off-white to light yellow crystalline powder
- Melting Point:92-96 °C(lit.)
- Refractive Index:1.59
- Boiling Point:342.4 °C at 760 mmHg
- Flash Point:160.9 °C
- PSA:40.86000
- Density:1.56 g/cm3
- LogP:2.13588
4-CYANOPHENACYL BROMIDE(Cas 20099-89-2) Usage
|
Chemical Properties |
off-white to light yellow crystalline powder |
|
Uses |
4-(2-Bromoacetyl)benzonitrile is useful for the irreversible inhibitory activity of Glycogen synthase kinase 3 (GSK-3). Phenylhalomethylketones can be used in the study of novel GSK-3 inhibitors. |
|
General Description |
2-Bromo-4′-cyanoacetophenone can be synthesized from ethylbenzene via aerobic photooxidation using aqueous HBr. |
InChI:InChI=1/C10H7BrO/c1-2-8-3-5-9(6-4-8)10(12)7-11/h1,3-6H,7H2
20099-89-2 Relevant articles
Unexpected ritter reaction during acid-promoted 1,3-dithiol-2-one formation
Dumur, Frederic,Mayer, Cedric R.
, p. 889 - 896 (2013)
A series of aryl-substituted 1,3-dithiol...
Electroselective α-bromination of acetophenone using: In situ bromonium ions from ammonium bromide
Jagatheesan,Joseph Santhana Raj,Lawrence,Christopher
, p. 35602 - 35608 (2016)
A greener and expeditious method for the...
High Chemo-/Stereoselectivity for Synthesis of Polysubstituted Monofluorinated Pyrimidyl Enol Ether Derivatives
Kang, Lei,Wang, Fang,Zhang, Jinlong,Yang, Huameng,Xia, Chungu,Qian, Jinlong,Jiang, Gaoxi
supporting information, p. 1669 - 1674 (2021/03/08)
A novel intramolecular Smiles rearrangem...
Base-Catalyzed Intramolecular Defluorination/O-Arylation Reaction for the Synthesis of 3-Fluoro-1,4-oxathiine 4,4-Dioxide
Kang, Lei,Zhang, Jinlong,Yang, Huameng,Qian, Jinlong,Jiang, Gaoxi
supporting information, p. 785 - 789 (2021/04/09)
A novel process involving base-catalyzed...
An umpolung oxa-[2,3] sigmatropic rearrangement employing arynes for the synthesis of functionalized enol ethers
Gaykar, Rahul N.,George, Malini,Guin, Avishek,Bhattacharjee, Subrata,Biju, Akkattu T.
supporting information, p. 3447 - 3452 (2021/05/04)
An oxa-[2,3] sigmatropic rearrangement i...
Flexible on-site halogenation paired with hydrogenation using halide electrolysis
Shang, Xiao,Liu, Xuan,Sun, Yujie
supporting information, p. 2037 - 2043 (2021/03/26)
Direct electrochemical halogenation has ...
20099-89-2 Process route
-
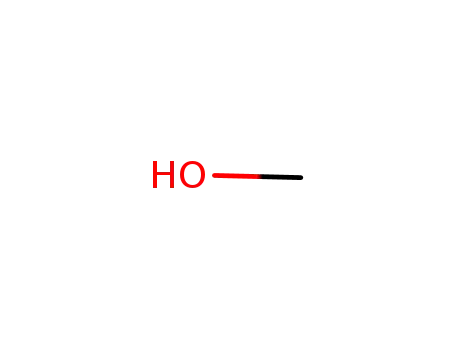
- 67-56-1
methanol

-
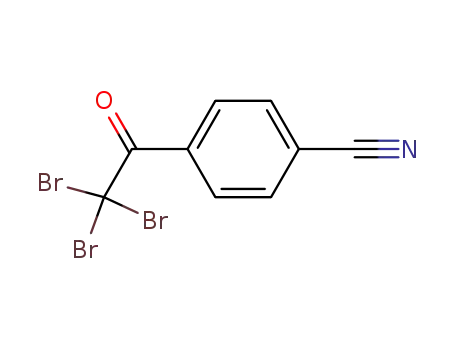
-
4-(2,2,2-tribromoacetyl)benzonitrile

-
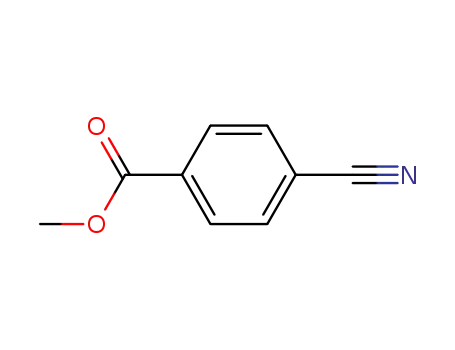
- 1129-35-7
4-cyanobenzoic acid methyl ester

-
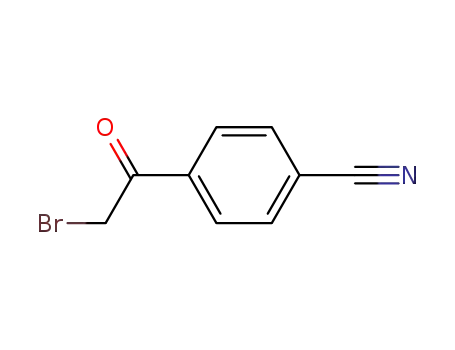
- 20099-89-2
4-Cyanophenacyl bromide

-
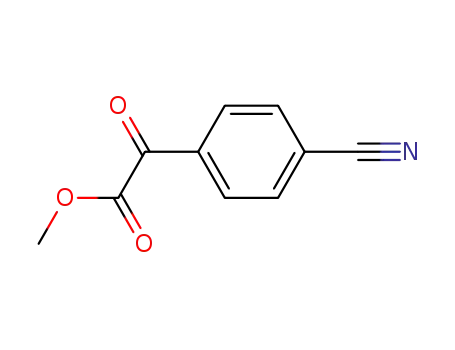
- 52798-47-7
methyl (4-cyanophenyl)glyoxylate

-
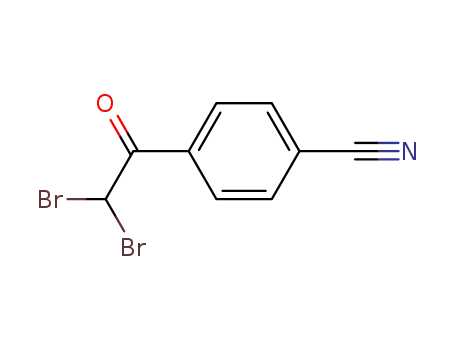
- 21661-87-0
2,2-dibromo-1-(4-cyanophenyl)ethan-1-one
| Conditions | Yield |
|---|---|
|
With oxygen; at 10 ℃; for 2h; Irradiation;
|
3.5 % Chromat. 5.4 % Chromat. 1 % Chromat. 59.0 % Chromat. |
-

- 25309-65-3
4-ethylbenzonitrile

-
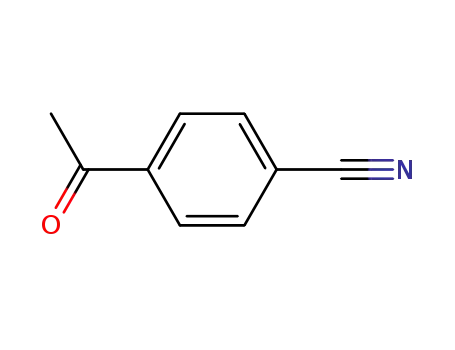
- 1443-80-7
4-cyanophenyl methyl ketone

-

- 20099-89-2
4-Cyanophenacyl bromide
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); water; In acetonitrile; at 80 ℃; for 8h;
|
20099-89-2 Upstream products
-
1443-80-7

4-cyanophenyl methyl ketone
-
67-56-1

methanol
-
25309-65-3

4-ethylbenzonitrile
-
157729-39-0
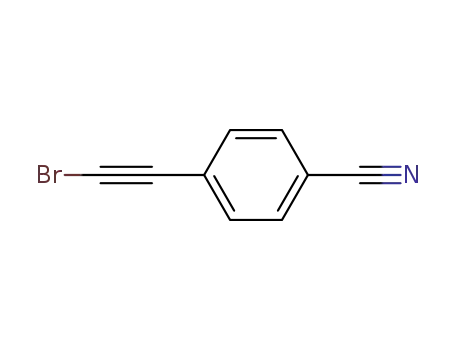
4-(2-bromoethynyl)-benzonitrile
20099-89-2 Downstream products
-
100537-59-5
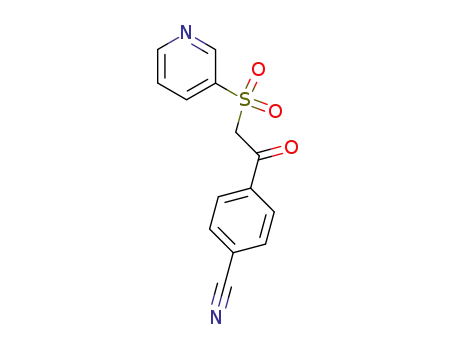
4-[(pyridine-3-sulfonyl)-acetyl]-benzonitrile
-
103962-24-9
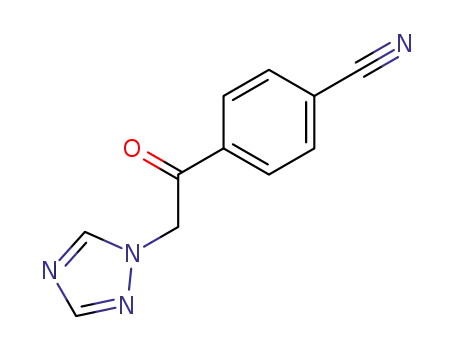
4-(2-[1,2,4]triazol-1-ylacetyl)benzonitrile
-
94020-34-5
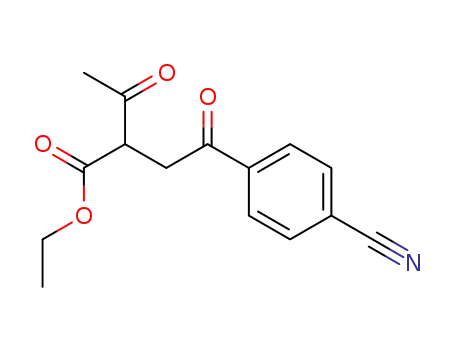
ethyl 2-acetyl-4-(4-cyanophenyl)-4-oxobutanoate
-
138470-03-8
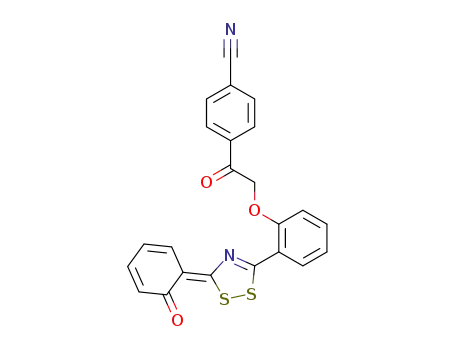
6-<5-<2-(4-Cyanphenacyloxy)-phenyl>-1,2,4-dithiazol-3-yliden>-2,4-cyclohexadien-1-on
相关产品
-
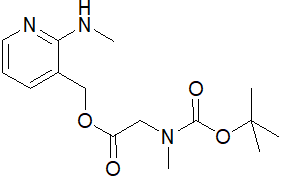
(2-(甲基氨基)吡啶-3-基)甲基 2-((叔丁氧羰基)(甲基)氨基)乙酸酯
CAS:1180002-01-0
-
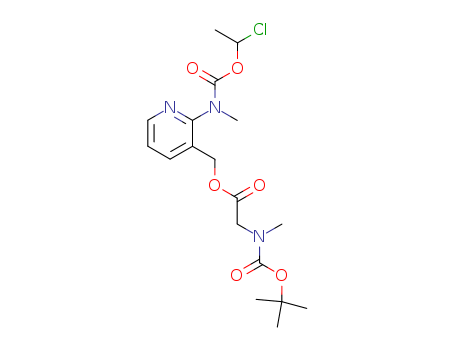
N-甲基-N-(3-[((N-叔丁氧羰基-N-甲基氨基)乙酰氧基)甲基]吡啶-2-基)氨基甲酸(1-氯乙基)酯
CAS:338990-31-1
-
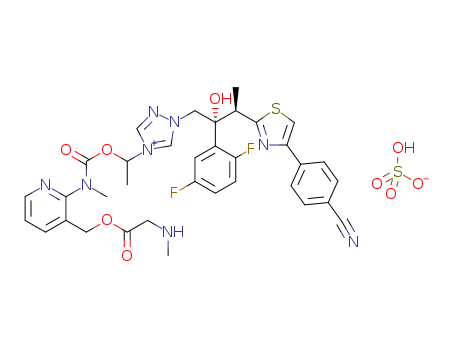
艾沙康唑硫酸盐
CAS:946075-13-4