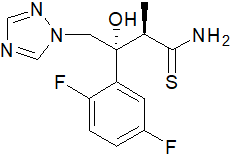
产品详情
pd_meltingpoint:64-66°C
- Molecular Formula:C22H17F2N5OS
- Molecular Weight:437.473
- Vapor Pressure:3.89E-19mmHg at 25°C
- Melting Point:64-66°C
- Boiling Point:674.9°C at 760 mmHg
- PKA:11.62±0.29(Predicted)
- Flash Point:362°C
- PSA:115.86000
- Density:1.38g/cm3
- LogP:4.24298
4-[2-[(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)b utan-2-yl]-1,3-thiazol-4-yl]benzonitrile(Cas 182760-06-1) Usage
|
Description |
Ravuconazole is an orally available triazole fungicide that potently inhibits the growth of a wide range of fungi (MICs range from 25 to 780 ng/ml). Like other azoles, ravuconazole inhibits cytochrome P450 (CYP) isoforms that are involved in ergosterol biosynthesis, interfering with the generation of the fungal and protozoan cell membranes. Ravuconazole specifically inhibits sterol 14α-demethylase (CYP51). As this enzyme is also important in the development of trypanosomes, ravuconazole is effective against T. cruzi infections in animal models of Chagas disease. |
|
Chemical Properties |
Off-White Solid |
|
Uses |
Ergosterol biosynthesis inhibitor. Antifungal. |
InChI:InChI=1/C22H17F2N5OS/c1-14(21-28-20(10-31-21)16-4-2-15(9-25)3-5-16)22(30,11-29-13-26-12-27-29)18-7-6-17(23)8-19(18)24/h2-8,10,12-14,30H,11H2,1H3/t14-,22+/m0/s1
182760-06-1 Relevant articles
AN IMPROVED PROCESS FOR THE PREPARATION OF TRIAZOLE DERIVATIVES
-
Page/Page column 22-23, (2021/12/31)
The present invention relates to an impr...
An enantioselective synthesis of the key intermediate for triazole antifungal agents; Application to the catalytic asymmetric synthesis of efinaconazole (jublia)
Tamura, Keiji,Kumagai, Naoya,Shibasaki, Masakatsu
supporting information, p. 3272 - 3278 (2014/05/06)
A new synthetic route, the shortest repo...
Process for the manufacture of enantiomerically pure antifungal azoles as ravuconazole and isavuconazole
-
Page/Page column 7-8, (2011/04/25)
A new technical process for preparation ...
Improved chiral synthesis of ravuconazole
Xu, Lin,Muller, Marc R.,Yu, Xiong,Zhu, Bao-Quan
experimental part, p. 1611 - 1625 (2009/10/17)
A short, elegant, and high yielding synt...
182760-06-1 Process route
-
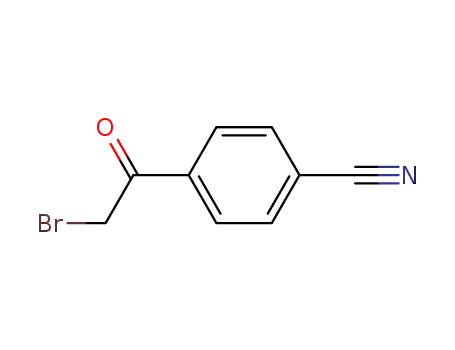
- 20099-89-2
4-Cyanophenacyl bromide

-
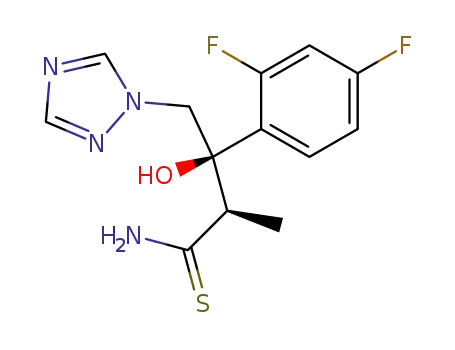
- 170863-34-0
(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-2-methyl-4-(1H-1,2,4-triazol-1-yl)butanethioamide

-
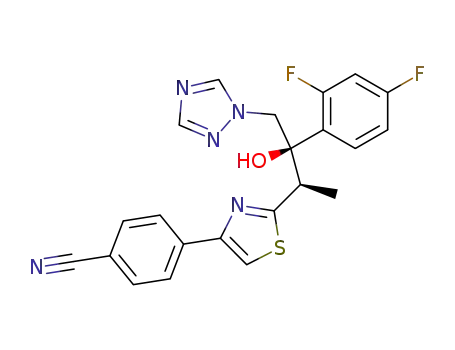
- 182760-06-1
ravuconazole
| Conditions | Yield |
|---|---|
|
In ethanol; for 3h; Reflux;
|
79.5% |
|
In ethanol; at 20 - 70 ℃; for 2h; enantioselective reaction; Inert atmosphere;
|
78% |
|
In ethanol; for 2h; Heating;
|
38% |
|
In methanol; at 60 - 65 ℃; for 3h;
|
120 g |
|
In methanol; at 60 - 65 ℃; for 3h;
|
120 g |
-
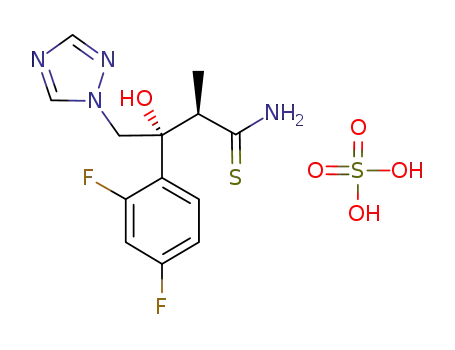
- 1175536-51-2
(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-2-methyl-4-(1H-1,2,4-triazol-1-yl)butanethioamide hydrosulfate

-

- 20099-89-2
4-Cyanophenacyl bromide

-

- 182760-06-1
ravuconazole
| Conditions | Yield |
|---|---|
|
(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-2-methyl-4-(1H-1,2,4-triazol-1-yl)butanethioamide hydrosulfate; 4-Cyanophenacyl bromide; In ethanol; water; at 60 - 70 ℃; for 2h; Large scale reaction;
With triethylamine; In ethanol; water; at 55 - 70 ℃; pH=3.5 - 4; Large scale reaction;
|
85.8% |
182760-06-1 Upstream products
-
20099-89-2

4-Cyanophenacyl bromide
-
170863-34-0

(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-2-methyl-4-(1H-1,2,4-triazol-1-yl)butanethioamide
-
1443-80-7
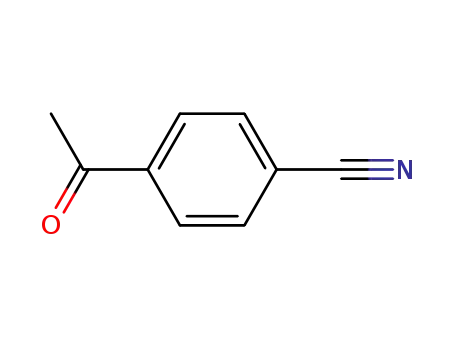
4-cyanophenyl methyl ketone
-
170862-36-9
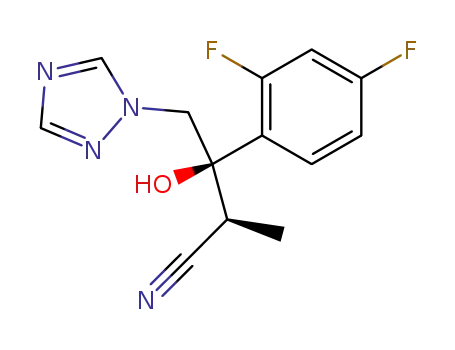
(2S,3R)-3-(2,4-difluorophenyl)-3-hydroxy-2-methyl-4-(1H-1,2,4-triazol-1-yl)butanenitrile
182760-06-1 Downstream products
-
368421-60-7
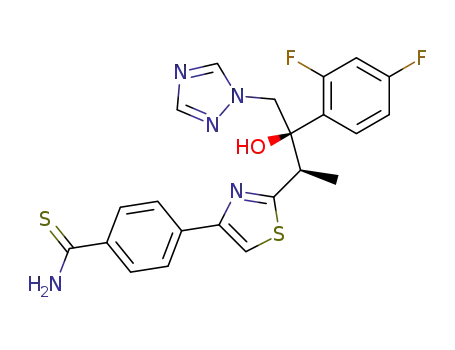
4-{2-[(1R,2R)-2-(2,4-Difluoro-phenyl)-2-hydroxy-1-methyl-3-[1,2,4]triazol-1-yl-propyl]-thiazol-4-yl}-thiobenzamide
-
351227-63-9
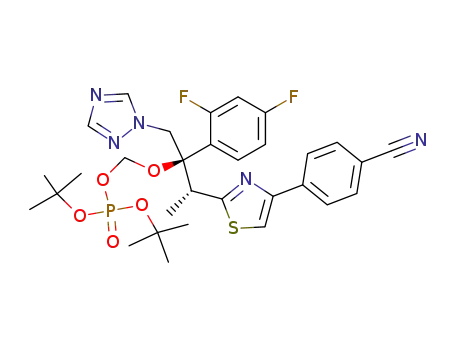
di-tert-butyl-[{(1R,2R)-2-[4-(4-cyanophenyl)-1,3-thiazol-2-yl]-1-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-ylmethyl)propyl}-oxy]methyl phosphate
-
207224-83-7
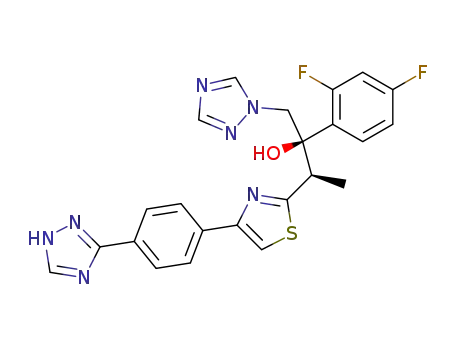
(2R,3R)-2-(2,4-Difluoro-phenyl)-1-[1,2,4]triazol-1-yl-3-{4-[4-(1H-[1,2,4]triazol-3-yl)-phenyl]-thiazol-2-yl}-butan-2-ol
-
251295-70-2
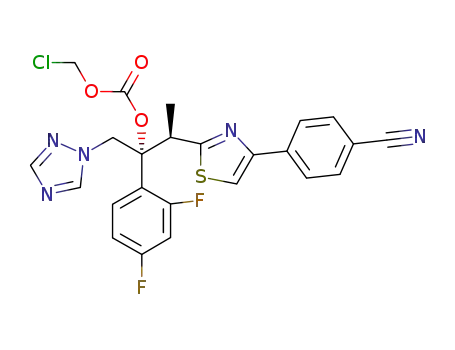
carbonic acid chloromethyl ester 2-[4-(4-cyanophenyl)-thiazol-2-yl]-1-(2,4-difluorophenyl)-1-[1,2,4]triazol-1-yl-methylpropyl ester
相关产品
-
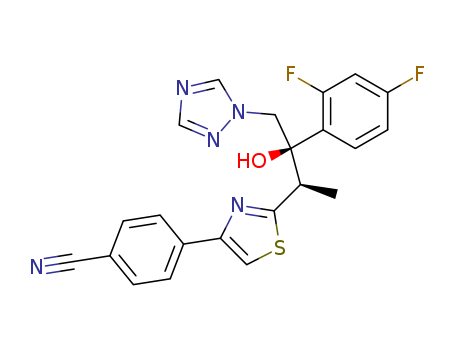
(2R,3R)-3-(2,5-二氟苯基)-3-羟基-2-甲基-4-(1H-1,2,4-三唑-1-基)硫代丁酰胺
CAS:368421-58-3
-
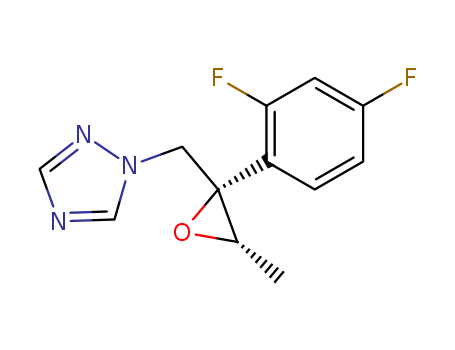
1-(((2R,3S)-2-(2,4-二氟苯基)-3-甲基环氧乙基-2-基)甲基)-1H-1,2,4-三唑
CAS:127000-90-2
-
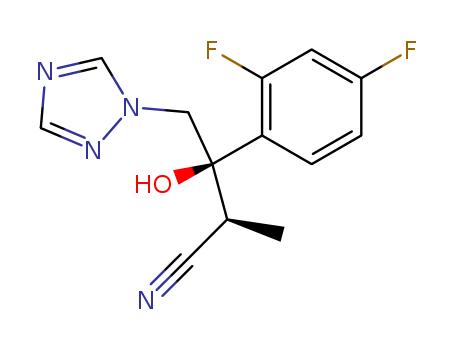
(ALPHAS,BETAR)-BETA-(2,4-二氟苯基)-BETA-羟基-ALPHA-甲基-1H-1,2,4-三唑-1-丁腈
CAS:170862-36-9