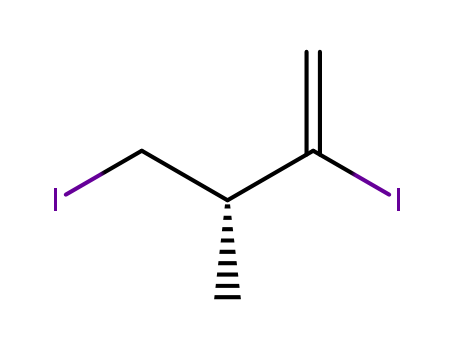
产品详情
- Molecular Formula:C5H8I2
- Molecular Weight:321.928
- Boiling Point:207.2±33.0 °C(Predicted)
- PSA:0.00000
- Density:2.235±0.06 g/cm3(Predicted)
- LogP:3.00620
481048-22-0 Relevant articles
ERP ((3R)-2,4-diiodo-3-N-methyl butylamine-1-ene) compound as well as preparation method and application thereof
-
Paragraph 0043-0059, (2019/08/03)
The invention provides an ERP ((3R)-2,4-...
(3R)-2-iodo-4-benzyloxy-3-methyl-1-ene compound as well as preparation method and application thereof
-
Paragraph 0056; 0057; 0058, (2019/08/02)
The invention provides a (3R)-2-iodo-4-b...
FE/CU-MEDIATED KETONE SYNTHESIS
-
, (2019/01/22)
Provided herein are methods for preparin...
Method for preparing eribulin and intermediate thereof
-
Paragraph 0047-0048, (2018/09/08)
The present invention relates to a metho...
481048-22-0 Process route
-
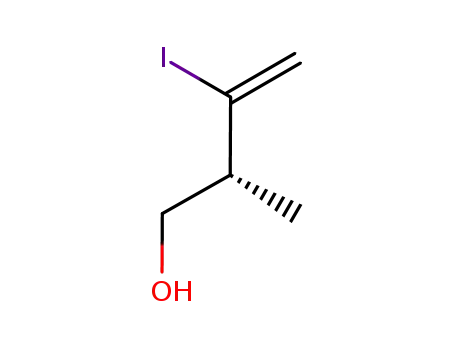
- 1193377-17-1
C5H9IO

-
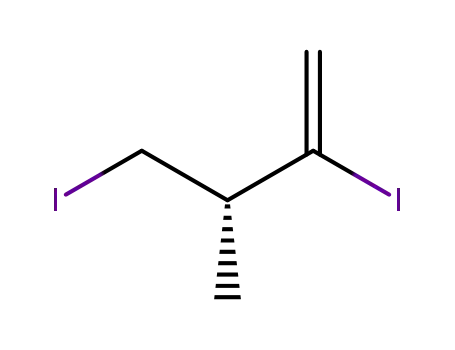
- 481048-22-0
(3R)-2,4-diiodo-3-methylbut-1-ene
Conditions
| Conditions | Yield |
|---|---|
|
With 1H-imidazole; iodine; triphenylphosphine; In tetrahydrofuran; at 0 - 55 ℃; for 2h; Temperature; Solvent; Reagent/catalyst; Inert atmosphere; Large scale;
|
97.1% |
|
With 1H-imidazole; iodine; triphenylphosphine; In tetrahydrofuran; at 0 - 55 ℃; for 2h; Inert atmosphere; Large scale;
|
95% |
|
With 1H-imidazole; iodine; triphenylphosphine; In dichloromethane; at 20 ℃; for 12h;
|
86% |
|
With 1H-imidazole; iodine; triphenylphosphine; Inert atmosphere;
|
76% |
|
Multi-step reaction with 2 steps
1: triethylamine; dmap / dichloromethane / 16 h / Heating
2: sodium iodide / acetone / 16 h / 50 °C
With dmap; triethylamine; sodium iodide; In dichloromethane; acetone;
|
|
|
With 1H-imidazole; iodine; triphenylphosphine; In toluene; acetonitrile; at 30 - 55 ℃; Reagent/catalyst; Solvent; Temperature; Time;
|
21.2 g |
|
Multi-step reaction with 2 steps
1: triethylamine / dichloromethane / 8 h / 10 - 20 °C / Inert atmosphere
2: sodium iodide / acetone / 25 h / 45 °C / Inert atmosphere
With triethylamine; sodium iodide; In dichloromethane; acetone;
|
-
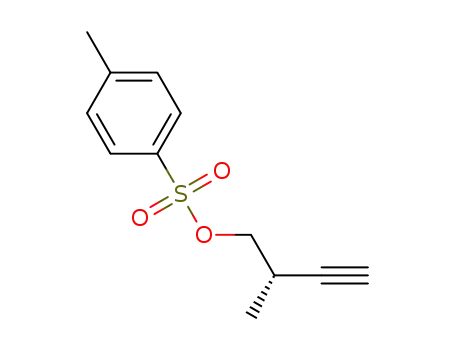
-
(2S)-2-methylbut-3-yn-1-yl-4-methylbenzene-1-sulfonate

-

- 481048-22-0
(3R)-2,4-diiodo-3-methylbut-1-ene
Conditions
| Conditions | Yield |
|---|---|
|
With chloro-trimethyl-silane; lithium iodide; In acetonitrile; at 20 ℃; for 10h;
|
87% |
|
Multi-step reaction with 2 steps
1: sodium bromide / acetonitrile / 20 h / 20 °C
2: lithium iodide; chloro-trimethyl-silane / acetonitrile / 10 h / 20 °C
With chloro-trimethyl-silane; sodium bromide; lithium iodide; In acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: lithium iodide / acetonitrile / 20 h / 20 °C
2: sodium iodide; chloro-trimethyl-silane / acetonitrile / 12.5 h / 20 °C
With chloro-trimethyl-silane; sodium iodide; lithium iodide; In acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: sodium bromide / acetonitrile / 20 h / 20 °C
2: lithium iodide / acetonitrile / 20 h / 20 °C
3: sodium iodide; chloro-trimethyl-silane / acetonitrile / 12.5 h / 20 °C
With chloro-trimethyl-silane; sodium iodide; sodium bromide; lithium iodide; In acetonitrile;
|
|
|
Multi-step reaction with 2 steps
1: sodium iodide; chloro-trimethyl-silane / acetonitrile / 7 h / 30 °C
2: sodium iodide / butanone / 18 h / 90 °C
With chloro-trimethyl-silane; sodium iodide; In acetonitrile; butanone;
|
481048-22-0 Upstream products
-
1193377-17-1

C5H9IO
-
337375-93-6
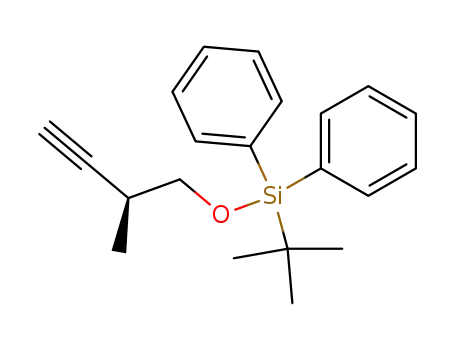
(S)-tert-butyl((2-methylbut-3-yn-1-yl)oxy)diphenylsilane
-
1442105-07-8
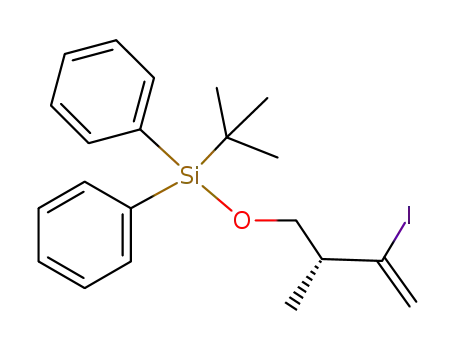
(R)-tert-butyl((3-iodo-2-methylbut-3-en-1-yl)oxy)diphenylsilane
-
1442105-08-9
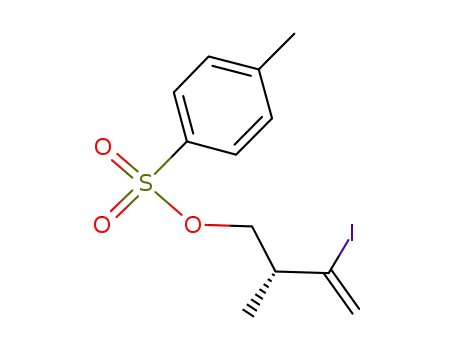
C12H15IO3S
481048-22-0 Downstream products
-
157322-47-9
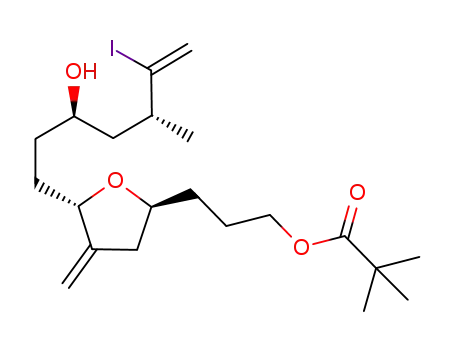
3-((2S,5S)-5-((3R,5R)-3-hydroxy-6-iodo-5-methylhept-6-en-1-yl)-4-methylenetetrahydrofuran-2-yl)propyl pivalate