871348-24-2
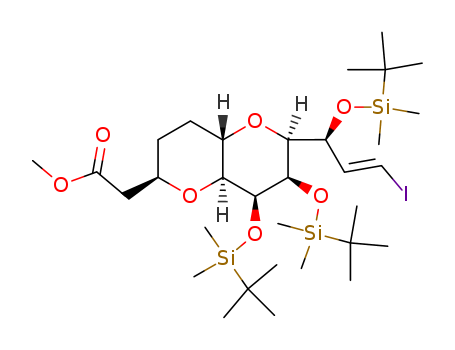
- 产品名称:2-((2S,3S,4R,5R)-5-((S)-2,3-二((叔丁基二甲基硅基)氧)丙基)-4-甲氧基-3-(苯磺酰基甲基)四氢呋喃-2-基)乙醛
- 分子式:C29H52O7SSi2
- 纯度:99%
- 分子量:600.965
产品详情
- Molecular Formula:C29H52O7SSi2
- Molecular Weight:600.965
- Boiling Point:597.9±48.0 °C(Predicted)
- PSA:96.51000
- Density:1.07±0.1 g/cm3(Predicted)
- LogP:7.33100
871348-24-2 Relevant articles
Process development of halaven: Synthesis of the C14-C35 fragment via iterative nozaki-hiyama-kishi reaction-williamson ether cyclization
Austad, Brian C.,Benayoud, Farid,Calkins, Trevor L.,Campagna, Silvio,Chase, Charles E.,Choi, Hyeong-Wook,Christ, William,Costanzo, Robert,Cutter, James,Endo, Atsushi,Fang, Francis G.,Hu, Yongbo,Lewis, Bryan M.,Lewis, Michael D.,McKenna, Shawn,Noland, Thomas A.,Orr, John D.,Pesant, Marc,Schnaderbeck, Matthew J.,Wilkie, Gordon D.,Abe, Taichi,Asai, Naoki,Asai, Yumi,Kayano, Akio,Kimoto, Yuichi,Komatsu, Yuki,Kubota, Manabu,Kuroda, Hirofumi,Mizuno, Masanori,Nakamura, Taiju,Omae, Takao,Ozeki, Naoki,Suzuki, Taeko,Takigawa, Teiji,Watanabe, Tomohiro,Yoshizawa, Kazuhiro
, p. 327 - 332 (2013/04/10)
Multikilogram manufacturing process of t...
INTERMEDIATES FOR THE PREPARATION OF HALICHONDRIN B
-
Page/Page column 41, (2010/02/15)
The present invention provides macrocycl...
871348-24-2 Process route
-
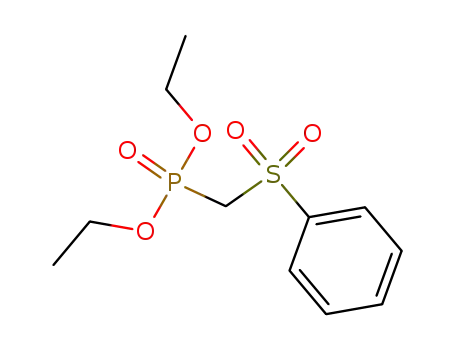
- 56069-39-7
diethyl phenylsulfonylmethylphosphonate

-
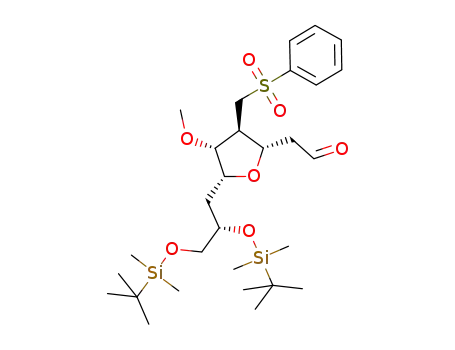
- 871348-24-2
2-((2S,3S,4R,5R)-5-((S)-2,3-bis((tert-butyldimethylsilyl)oxy)propyl)-4-methoxy-3-((phenylsulfonyl)methyl)tetrahydrofuran-2-yl)acetaldehyde
Conditions
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 9 steps
1.1: lithium hexamethyldisilazane / toluene; tetrahydrofuran / 0 - 20 °C
2.1: trimethylsilyl iodide / toluene; acetonitrile / 60 °C
3.1: sodium tris(acetoxy)borohydride; tetrabutyl-ammonium chloride / toluene; 1,2-dimethoxyethane / 80 °C
4.1: potassium carbonate / methanol / 50 °C
5.1: sulfuric acid / 1,2-dimethoxyethane / 40 °C
6.1: sodium t-butanolate / 1,2-dimethoxyethane; 1-methyl-pyrrolidin-2-one / 10 °C
7.1: hydrogenchloride / methanol / 25 °C
8.1: 1H-imidazole / N,N-dimethyl-formamide / 25 °C
9.1: ozone / isopropyl alcohol / 50 °C
9.2: Lindlar’s catalyst / 15 °C
With 1H-imidazole; hydrogenchloride; trimethylsilyl iodide; sulfuric acid; tetrabutyl-ammonium chloride; sodium tris(acetoxy)borohydride; potassium carbonate; ozone; lithium hexamethyldisilazane; sodium t-butanolate; In tetrahydrofuran; 1-methyl-pyrrolidin-2-one; methanol; 1,2-dimethoxyethane; N,N-dimethyl-formamide; isopropyl alcohol; toluene; acetonitrile;
|
-
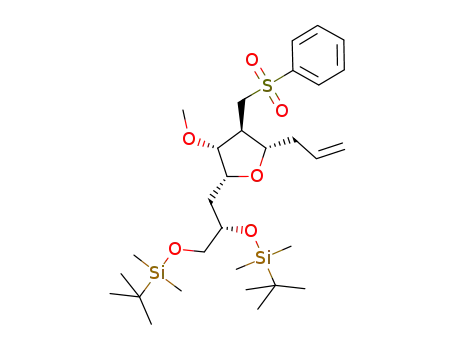
- 871348-22-0
C30H54O6SSi2

-

- 871348-24-2
2-((2S,3S,4R,5R)-5-((S)-2,3-bis((tert-butyldimethylsilyl)oxy)propyl)-4-methoxy-3-((phenylsulfonyl)methyl)tetrahydrofuran-2-yl)acetaldehyde
Conditions
| Conditions | Yield |
|---|---|
|
C30H54O6SSi2; With ozone; In isopropyl alcohol; at 50 ℃;
With 2,6-di-tert-butyl-4-methyl-phenol; hydrogen; In isopropyl alcohol; at 15 ℃;
|
89% |
|
C30H54O6SSi2; With ozone; In n-heptane; at -60 - 5 ℃; for 0.25 - 0.5h;
With hydrogen; 5% Pd on CaCO3 poisoned with Pb; In n-heptane; at 20 - 25 ℃; for 2.5h; under 760.051 Torr;
|
871348-24-2 Upstream products
-
871348-22-0

C30H54O6SSi2
-
56069-39-7

diethyl phenylsulfonylmethylphosphonate
-
871348-03-7
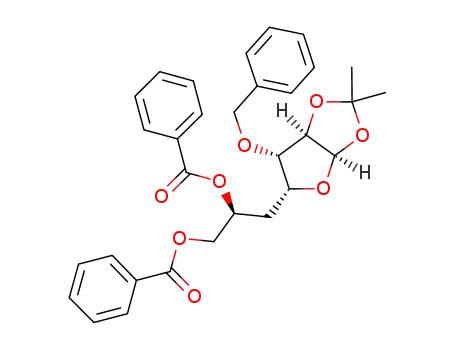
C31H32O8
-
546141-24-6
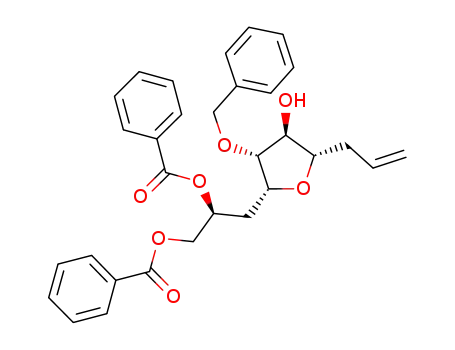
[(2S)-3-[(2R,3R,4S,5S)-5-allyl-3-benzyloxy-4-hydroxytetrahydrofuran-2-yl]-2-benzoyloxypropyl]benzoate
871348-24-2 Downstream products
-
1316279-29-4
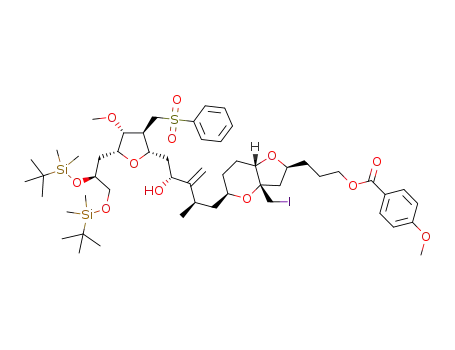
C53H85IO12SSi2
相关产品
-
(R)-2,4-二碘-3-甲基-1-丁烯
CAS:481048-22-0
-
磺达肝癸钠单糖
CAS:87907-36-6