产品详情
- Molecular Formula:C9H17NO2
- Molecular Weight:171.239
- Vapor Pressure:0.0835mmHg at 25°C
- Refractive Index:1.441
- Boiling Point:226.1 °C at 760 mmHg
- PKA:6.65±0.10(Predicted)
- Flash Point:90.5 °C
- PSA:38.33000
- Density:0.969 g/cm3
- LogP:1.26640
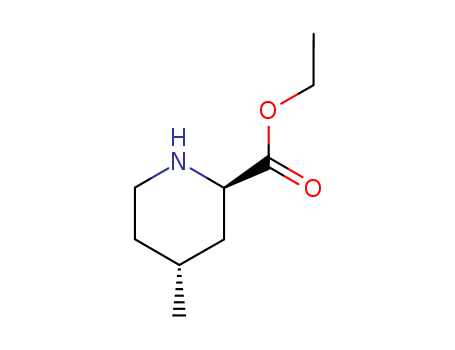
Ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate(Cas 74892-82-3) Usage
|
Chemical Properties |
light yellow solution |
|
Uses |
Ethyl (2R,4R)-4-Methylpipecolate is an intermediate in the preparation of Argatroban (A769000). |
InChI:InChI=1/C9H17NO2/c1-3-12-9(11)8-6-7(2)4-5-10-8/h7-8,10H,3-6H2,1-2H3/t7-,8-/m1/s1
74892-82-3 Relevant articles
Synthesis method of argatroban intermediate (2R,4R)-4-methyl piperidine-2-ethyl formate
-
Paragraph 0029; 0030; 0031; 0032-0034; 0039-0044; 0046; 0048, (2017/09/01)
The invention relates to a synthesis met...
PROCESS INTERMEDIATES AND METHODS FOR THE PREPARATION OF PROCESS INTERMEDIATES FOR THE SYNTHESIS OF ARGATROBAN MONOHYDRATE
-
Paragraph 0090, (2014/04/03)
Methods are provided for the synthesis o...
METHOD FOR THE PREPARATION OF PROCESS INTERMEDIATES FOR THE SYNTHESIS OF ARGATROBAN MONOHYDRATE
-
Page/Page column 59, (2012/10/18)
Object of the present invention is a met...
Diastereoselective synthesis of an argatroban intermediate, ethyl (2R,4R)-4-methylpipecolate, by means of a Mandyphos/rhodium complex-catalyzed hydrogenation
Ferraboschi, Patrizia,Mieri, Maria De,Grisenti, Paride,Lotz, Matthias,Nettekoven, Ulrike
experimental part, p. 1626 - 1631 (2012/01/03)
The synthetic antithrombotic argatroban ...
74892-82-3 Process route
-
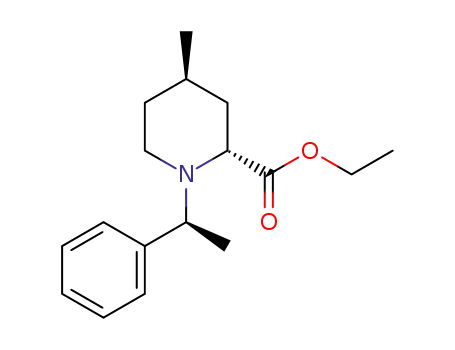
- 198641-56-4
ethyl (2R,4R)-4-methyl-1-((S)-1-phenylethyl)tetrahydropyridine-2-carboxylate

-
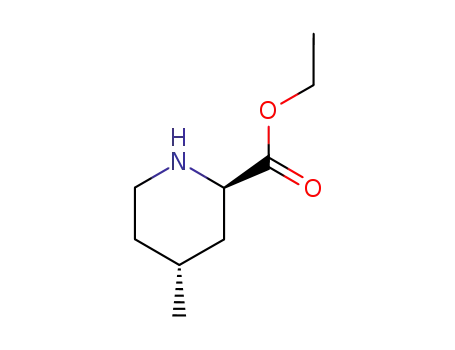
- 74892-82-3,42205-75-4
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate
| Conditions | Yield |
|---|---|
|
With 10% palladium hydroxide on charcoal; hydrogen; In ethanol; at 20 ℃; for 4h; under 760.051 Torr;
|
45% |
|
With palladium 10% on activated carbon; hydrogen; iron(II) oxalate; acetic acid; In ethanol; at 30 ℃; for 4h; under 3750.38 Torr; Reagent/catalyst; Autoclave;
|
345 g |
-
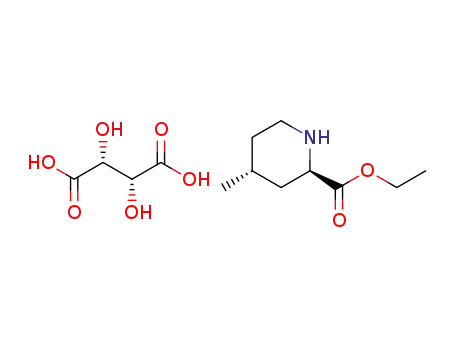
- 131278-84-7
ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate L-(+)-tartarate

-

- 74892-82-3,42205-75-4
ethyl (2R-trans)-4-methylpiperidine-2-carboxylate
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In water; ethyl acetate; at 15 - 20 ℃; for 1h;
|
88% |
74892-82-3 Upstream products
-
134984-62-6
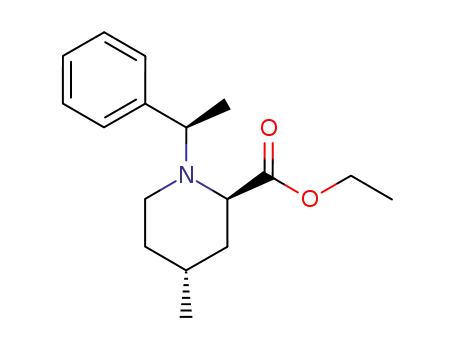
(2R,4R)-4-Methyl-1-((R)-1-phenyl-ethyl)-piperidine-2-carboxylic acid ethyl ester
-
139334-62-6
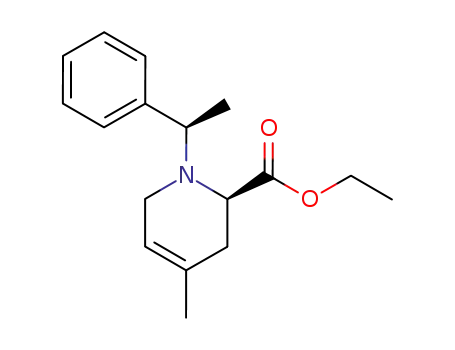
(6R)-1-<(R)-1-phenylethyl>-6-ethoxycarbonyl-4-methyl-3,4-didehydropiperidine
-
78-79-5
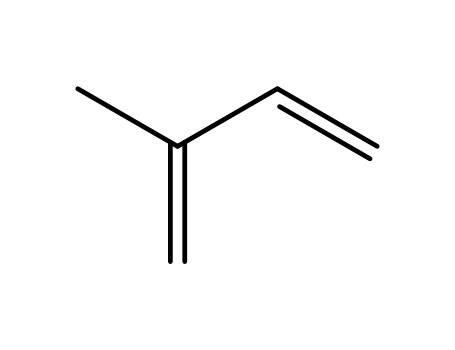
isoprene
-
139334-63-7
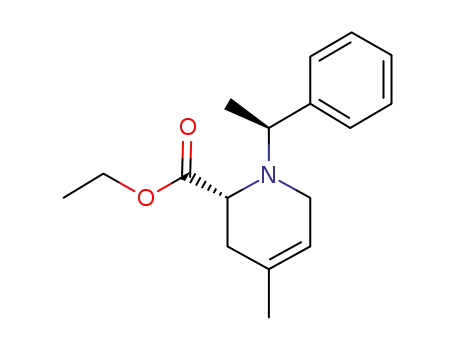
ethyl (2R)-4-methyl-1-((S)-1-phenylethyl)-1,2,3,6-tetrahydropyridine-2-carboxylate
74892-82-3 Downstream products
-
174699-01-5
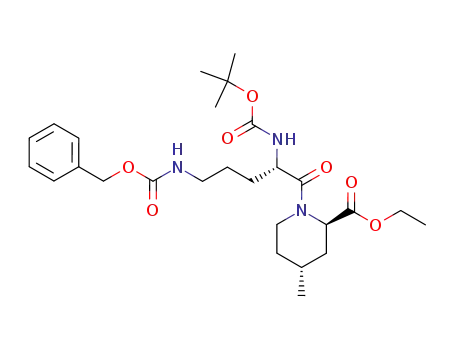
(2R,4R)-1-((S)-5-Benzyloxycarbonylamino-2-tert-butoxycarbonylamino-pentanoyl)-4-methyl-piperidine-2-carboxylic acid ethyl ester
-
183679-13-2
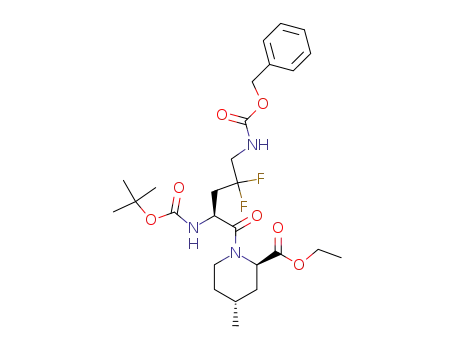
(2R,4R)-1-((S)-5-Benzyloxycarbonylamino-2-tert-butoxycarbonylamino-4,4-difluoro-pentanoyl)-4-methyl-piperidine-2-carboxylic acid ethyl ester
-
174699-02-6
(2R,4R)-1-((S)-2-Amino-5-benzyloxycarbonylamino-pentanoyl)-4-methyl-piperidine-2-carboxylic acid ethyl ester
-
174699-04-8
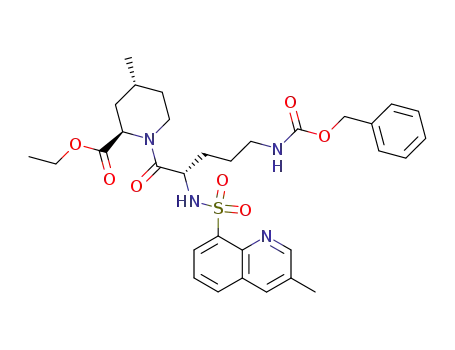
(2R,4R)-1-[(S)-5-Benzyloxycarbonylamino-2-(3-methyl-quinoline-8-sulfonylamino)-pentanoyl]-4-methyl-piperidine-2-carboxylic acid ethyl ester
相关产品
-
(2-(甲基氨基)吡啶-3-基)甲基 2-((叔丁氧羰基)(甲基)氨基)乙酸酯
CAS:1180002-01-0
-
(2R,4R)-4-甲基-2-哌啶甲酸
CAS:74892-81-2