产品详情
pd_meltingpoint:44-46 °C(lit.)
factory supply Manufacturer (1S,5R)-2-Oxabicyclo[3.3.0]oct-6-en-3-one 43119-28-4 Pharmaceutical Grade
- Molecular Formula:C7H8O2
- Molecular Weight:124.139
- Vapor Pressure:0.0105mmHg at 25°C
- Melting Point:44-46 °C(lit.)
- Refractive Index:1.4906 (estimate)
- Boiling Point:263.1oC at 760 mmHg
- Flash Point:104oC
- PSA:26.30000
- Density:1.196g/cm3
- LogP:0.87800
(1S,5R)-(-)-2-OXABICYCLO[3.3.0]OCT-6-EN-3-ONE(Cas 43119-28-4) Usage
|
Chemical Properties |
white to beige or brownish crystalline powder |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C7H8O2/c8-7-4-5-2-1-3-6(5)9-7/h1-2,5-6H,3-4H2/t5-,6-/m0/s1
43119-28-4 Relevant articles
Divorce in the two-component BVMO family: The single oxygenase for enantioselective chemo-enzymatic Baeyer-Villiger oxidations
R?llig, Robert,Paul, Caroline E.,Claeys-Bruno, Magalie,Duquesne, Katia,Kara, Selin,Alphand, Véronique
supporting information, p. 3441 - 3450 (2021/05/03)
Two-component flavoprotein monooxygenase...
Genome mining reveals new bacterial type I Baeyer-Villiger monooxygenases with (bio)synthetic potential
Bianchi, Dario A.,Carabajal, María Ayelén,Ceccoli, Romina D.,Rial, Daniela V.
, (2020/03/19)
Baeyer-Villiger monooxygenases (BVMOs) a...
Controlling the Regioselectivity of Baeyer–Villiger Monooxygenases by Mutation of Active-Site Residues
Balke, Kathleen,B?umgen, Marcus,Bornscheuer, Uwe T.
, p. 1627 - 1638 (2017/08/26)
Baeyer–Villiger monooxygenase (BVMO)-med...
Characterization and Crystal Structure of a Robust Cyclohexanone Monooxygenase
Romero, Elvira,Castellanos, J. Rubén Gómez,Mattevi, Andrea,Fraaije, Marco W.
supporting information, p. 15852 - 15855 (2016/12/16)
Cyclohexanone monooxygenase (CHMO) is a ...
43119-28-4 Process route
-
![bicyclo[3.2.0]hept-2-en-6-one](/upload/2023/9/f9d53361-6ad4-4a38-b6a7-b3f770e18cc7.png)
-
13173-09-6,62182-73-4,71155-04-9,71155-05-0
bicyclo[3.2.0]hept-2-en-6-one

-
![(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/3fd0324a-a8d1-40dd-be01-7bb512f6d680.png)
-
34638-25-0,38110-77-9,43119-28-4,54483-22-6,26054-46-6
(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
![(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/eff75376-3734-468a-b0cd-f83076eb1d42.png)
-
26054-46-6
(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
![3-oxabicyclo[3.3.0]oct-6-en-2-one](/upload/2023/9/695216a7-8c35-41b8-acf6-ce4885e9dfdd.png)
-
128946-78-1
3-oxabicyclo[3.3.0]oct-6-en-2-one

-
-
103618-27-5
(+)-(1S,5R)-3-oxabicyclo<3.3.0>oct-6-en-2-one
| Conditions | Yield |
|---|---|
|
With
oxygen;
In
water;
at 30 ℃;
for 2h;
Yield given. Yields of byproduct given;
biotransformation by Ps. putida NCIMB 10007 monooxygenase enzyme using NADPH as cofactor; further monooxygenases; pH 7.1;
|
|
|
With
Cumene hydroperoxide; (S)-[1,1']-binaphthalenyl-2,2'-diol; dimethylaluminum chloride;
In
hexane; toluene;
at -25 - 20 ℃;
for 12h;
Title compound not separated from byproducts;
|
|
|
With
2CF3O3S(1-)*C56H46O2P2Pt(2+); dihydrogen peroxide;
In
water;
at 5 ℃;
for 5h;
optical yield given as %ee;
enantioselective reaction;
|
|
|
With
secondary alcohol dehydrogenase; wild-type phenylacetone monooxygenase; NADP; isopropyl alcohol;
In
acetonitrile;
at 30 ℃;
pH=8;
optical yield given as %ee;
enantioselective reaction;
aq. buffer;
Enzymatic reaction;
|
|
|
With
C52H29O4P; dihydrogen peroxide;
In
dichloromethane; water;
at -40 ℃;
for 36h;
enantioselective reaction;
|
-
![bicyclo[3.2.0]hept-2-en-6-one](/upload/2023/9/f9d53361-6ad4-4a38-b6a7-b3f770e18cc7.png)
-
13173-09-6,62182-73-4,71155-04-9,71155-05-0
bicyclo[3.2.0]hept-2-en-6-one

-
![(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/3fd0324a-a8d1-40dd-be01-7bb512f6d680.png)
-
34638-25-0,38110-77-9,43119-28-4,54483-22-6,26054-46-6
(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
![(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/eff75376-3734-468a-b0cd-f83076eb1d42.png)
-
26054-46-6
(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
-
103618-27-5
(+)-(1S,5R)-3-oxabicyclo<3.3.0>oct-6-en-2-one
| Conditions | Yield |
|---|---|
|
With
oxygen;
In
water;
at 30 ℃;
for 1h;
Yield given. Title compound not separated from byproducts;
biotransformation by Ps. putida NCIMB 10007 monooxygenase enzyme using NADH as cofactor; further monooxygenases; pH 7.1;
|
47 % Chromat. |
|
With
oxygen;
In
water;
at 30 ℃;
for 1h;
Yields of byproduct given;
biotransformation by Ps. putida NCIMB 10007 monooxygenase enzyme using NADH as cofactor; further monooxygenases; pH 7.1;
|
47 % Chromat. |
|
With
phenylacetone monooxygenase Pro440Trp mutant; secondary alcohol dehydrogenase; NADP; isopropyl alcohol;
In
acetonitrile;
at 30 ℃;
pH=8;
optical yield given as %ee;
enantioselective reaction;
aq. buffer;
Enzymatic reaction;
|
|
|
With
D-glucose;
In
water; N,N-dimethyl-formamide;
at 20 ℃;
for 48h;
Reagent/catalyst;
regioselective reaction;
Enzymatic reaction;
|
43119-28-4 Upstream products
-
925211-06-9
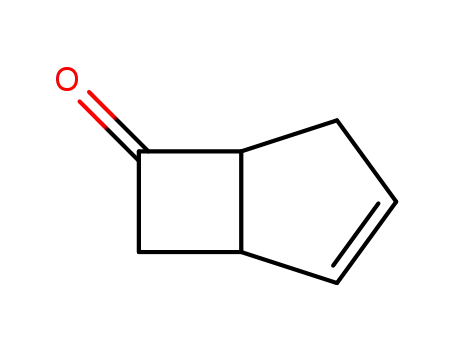
bicyclo(3.2.0)hept-2-en-6-one
-
71155-04-9
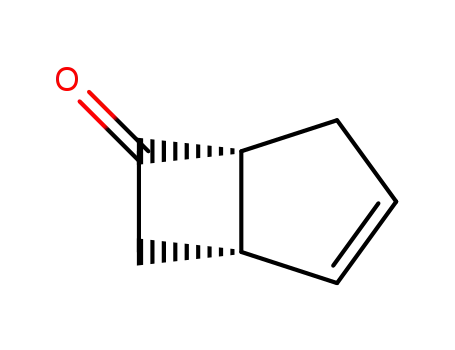
(1S,5R)-(-)-bicyclo[3.2.0]hept-2-en-6-one
-
71155-05-0
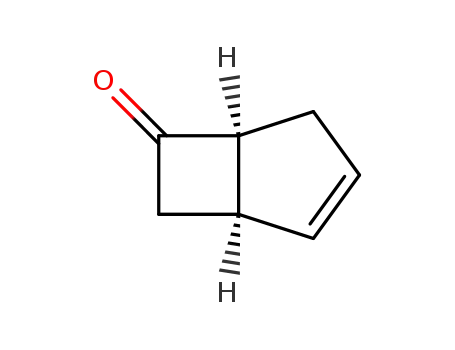
(1R,5S)-bicyclo[3.2.0]hept-2-en-6-one
-
13173-09-6
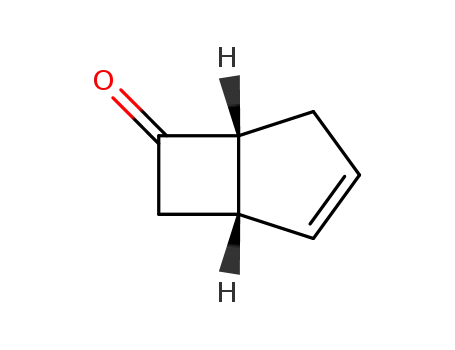
bicyclo[3.2.0]hept-2-en-6-one
43119-28-4 Downstream products
-
76704-05-7
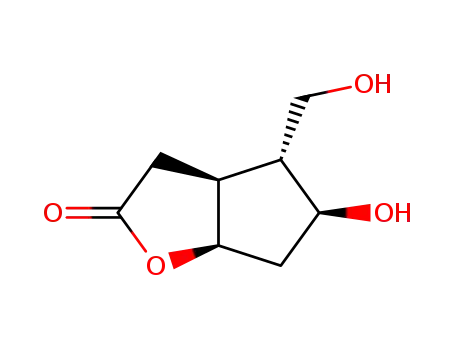
<3aS(3aα,4α,5β,6aα)>-(+)-5-hydroxy-4-hydroxymethyl-hexahydro-2H-cyclopentafuran-2-one
-
143741-39-3
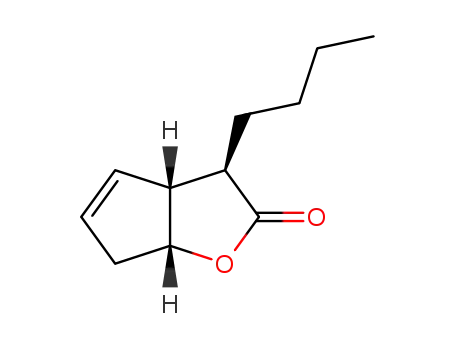
(3R,3aR,6aR)-3-Butyl-3,3a,6,6a-tetrahydro-cyclopenta[b]furan-2-one
-
143741-40-6
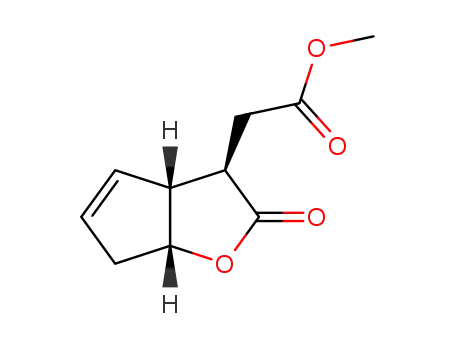
((3R,3aR,6aR)-2-Oxo-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan-3-yl)-acetic acid methyl ester
-
80040-30-8
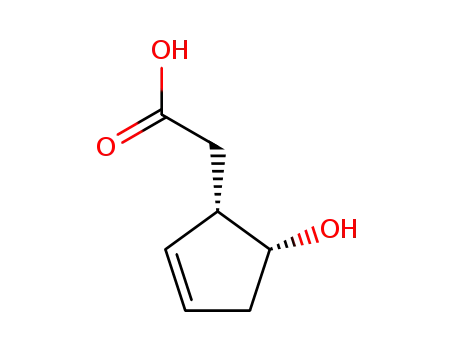
((1S,5R)-5-Hydroxy-cyclopent-2-enyl)-acetic acid
相关产品
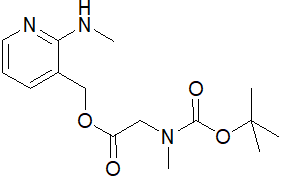
-
(2-(甲基氨基)吡啶-3-基)甲基 2-((叔丁氧羰基)(甲基)氨基)乙酸酯
CAS:1180002-01-0
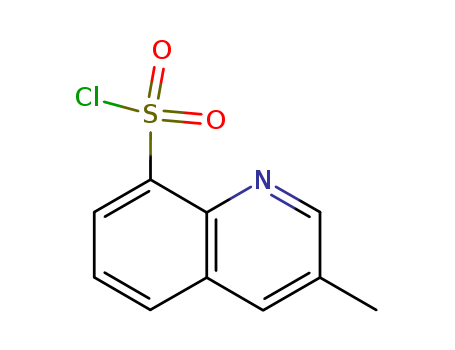
-
3-甲基-8-喹啉磺酰氯
CAS:74863-82-4
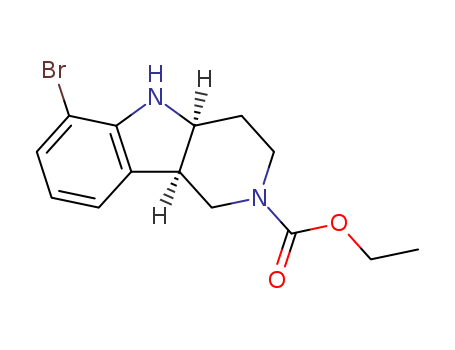
-
(4aS,9bR)-6-溴-3,4,4a,5-四氢-1H-吡啶并[4,3-b]吲哚-2(9bH)-羧酸乙酯
CAS:1059630-08-8