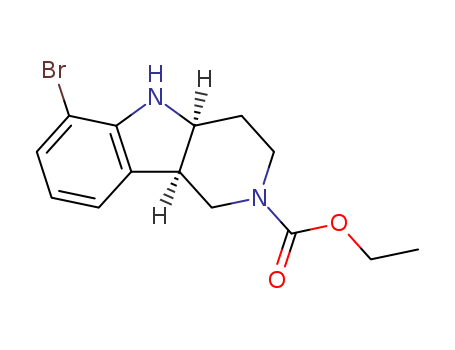
产品详情
- Molecular Formula:C14H17BrN2O2
- Molecular Weight:325.205
- Boiling Point:406.8±45.0 °C(Predicted)
- PKA:3.91±0.20(Predicted)
- Density:1.426±0.06 g/cm3(Predicted)
1059630-08-8 Relevant articles
Preparation method of Lumateperone intermediate
-
, (2021/06/26)
The invention discloses a preparation me...
SUBSTITUTED HETEROCYCLE FUSED GAMMA-CARBOLINES SYNTHESIS
-
Paragraph 00108, (2020/07/14)
The present invention provides improved ...
SUBSTITUTED HETEROCYCLE FUSED GAMMA-CARBOLINES SYNTHESIS
-
Paragraph 00102; 00103, (2020/07/14)
The present invention provides improved ...
SUBSTITUTED HETEROCYCLE FUSED GAMMA-CARBOLINES SYNTHESIS
-
Paragraph 000114-000115, (2020/01/08)
The present invention provides improved ...
1059630-08-8 Process route
-
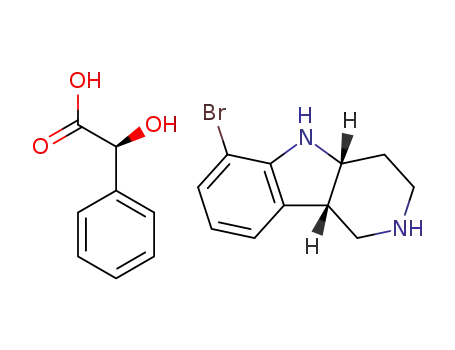
-
C11H13BrN2*C8H8O3

-
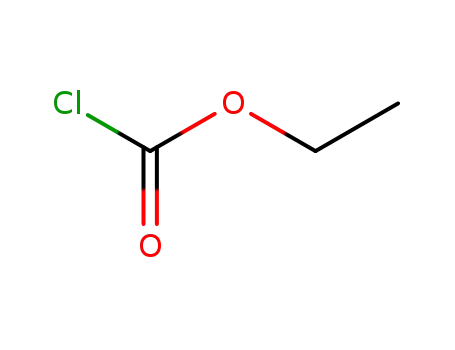
- 541-41-3
chloroformic acid ethyl ester

-
![(4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate](/upload/2023/9/6f4a44fd-37a5-42a5-aa86-f3d090bd0e48.png)
- 1059630-08-8
(4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In tetrahydrofuran; at 25 ℃; for 1.33333h; Inert atmosphere;
|
98% |
-
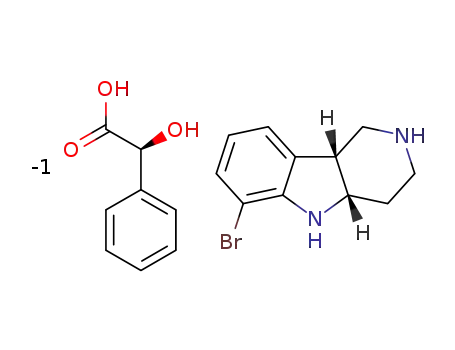
-
(x)C8H8O3*C11H13BrN2

-

- 541-41-3
chloroformic acid ethyl ester

-
![(4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate](/upload/2023/9/6f4a44fd-37a5-42a5-aa86-f3d090bd0e48.png)
- 1059630-08-8
(4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In tetrahydrofuran; at 25 ℃;
|
98% |
1059630-08-8 Upstream products
-
1059630-13-5
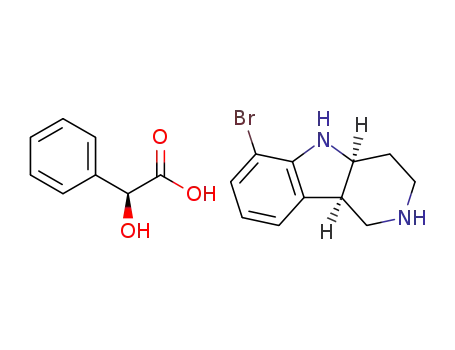
(4aS,9bR)-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole (S)-(+)-mandelic acid salt
-
541-41-3

chloroformic acid ethyl ester
-
1059630-07-7
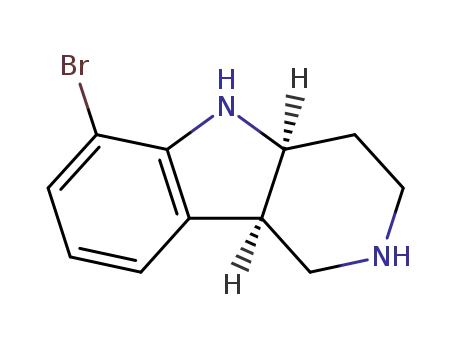
[4aS,9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole
-
50709-33-6
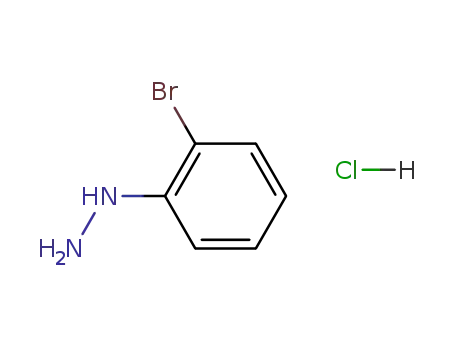
2-bromophenylhydrazine hydrochloride
1059630-08-8 Downstream products
-
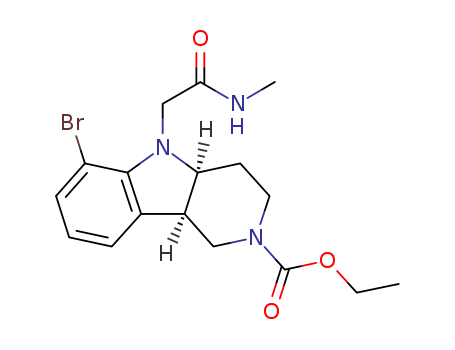
2098497-32-4
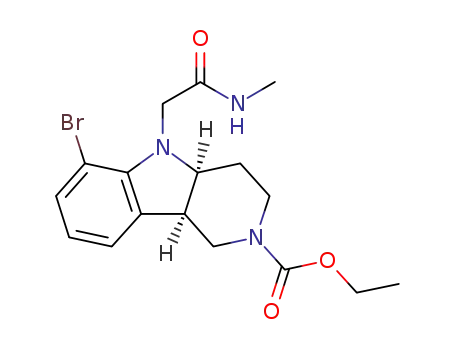
(4aS,9bR)-ethyl 6-bromo-5-(2-(methylamino)-2-oxoethyl)-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate
-
313369-25-4
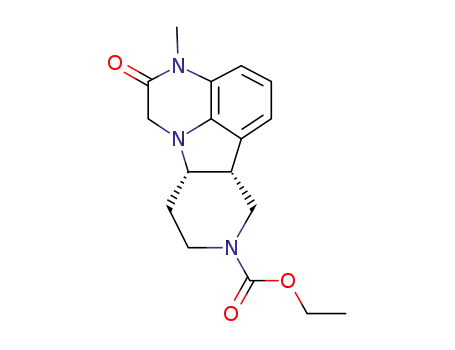
(6bR,10aS)-ethyl 2,3,6b,9,10,10a-hexahydro-3-methyl-2-oxo-1H-pyrido[3‘,4’:4,5]-pyrrolo[1,2,3-de]quinoxaline-8-carboxylate
-
313369-26-5
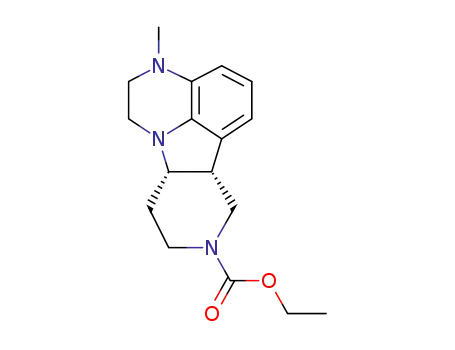
(6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3‘,4’:4,5]-pyrrolo[1,2,3-de]quinoxaline-8-carboxylate
-
313368-85-3
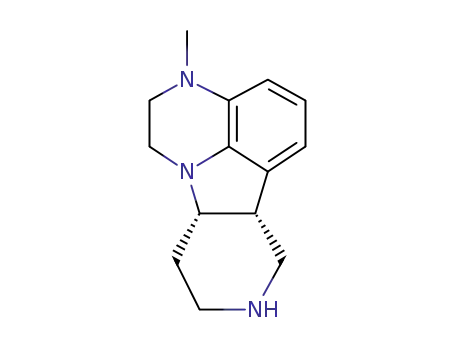
(6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido-[3’,4’:4,5]-pyrrolo[1,2,3-de]quinoxaline
相关产品
-
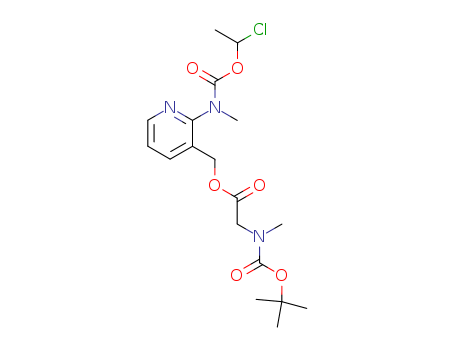
N-甲基-N-(3-[((N-叔丁氧羰基-N-甲基氨基)乙酰氧基)甲基]吡啶-2-基)氨基甲酸(1-氯乙基)酯
CAS:338990-31-1
-
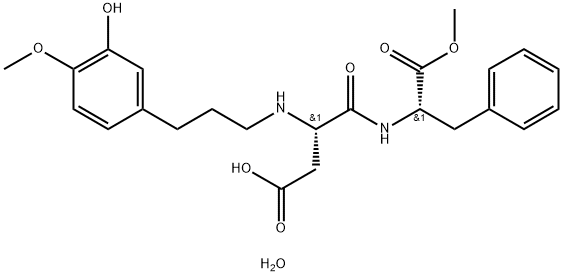
爱德万甜一水合物
CAS:714229-20-6
-
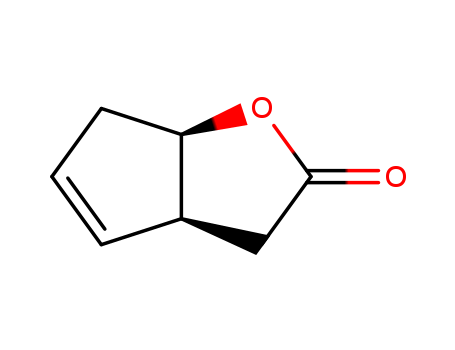
(1S,5R)-2-氧杂二环[3.3.0]辛-6-烯-3-酮
CAS:43119-28-4