2098497-32-4
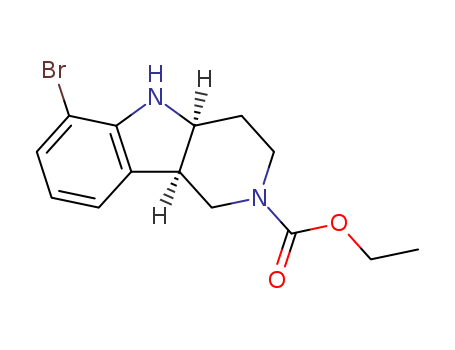
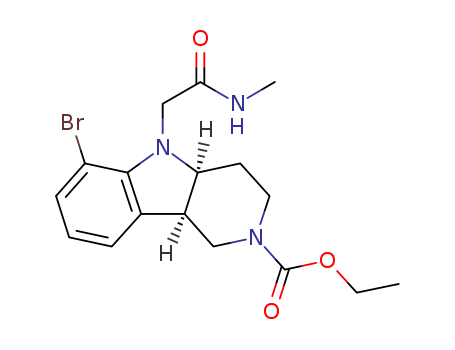
- 产品名称:(4AS,9BR)-6-溴-5-(2-(甲基氨基)-2-氧代乙基)-3,4,4A,5-四氢-1H-吡啶并[4,3-B]吲哚-2(9BH)-甲酸乙酯
- 分子式:
- 纯度:99%
- 分子量:396.284
产品详情
- Molecular Formula:C17H22BrN3O3
- Molecular Weight:396.284
- Boiling Point:540.0±50.0 °C(Predicted)
- Density:1.400±0.06 g/cm3(Predicted)
2098497-32-4 Relevant articles
SUBSTITUTED HETEROCYCLE FUSED GAMMA-CARBOLINES SYNTHESIS
-
Paragraph 000117; 000119, (2020/01/08)
The present invention provides improved ...
ITI-007
Cole
, p. 643 - 650 (2015/11/24)
A great deal has been achieved in the tr...
SUBSTITUTED HETEROCYCLE FUSED GAMMA-CARBOLINES SYNTHESIS
-
Page/Page column 87-90, (2008/12/07)
The present invention provides methods f...
2098497-32-4 Process route
-
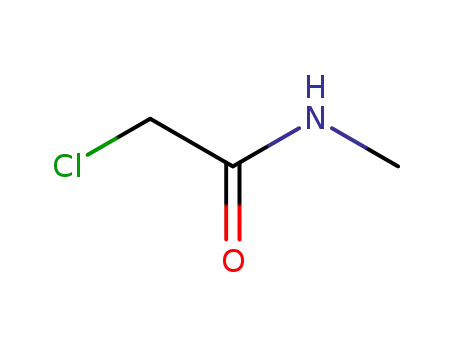
- 96-30-0
chloroacetylmethylamide

-
![(4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate](/upload/2023/9/14651167-5dd6-4367-9104-3fda18a0fd55.png)
- 1059630-08-8
(4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate

-
![(4aS,9bR)-ethyl 6-bromo-5-(2-(methylamino)-2-oxoethyl)-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/upload/2023/9/539ac3a6-91d2-449e-810f-3c9050eae495.png)
- 2098497-32-4
(4aS,9bR)-ethyl 6-bromo-5-(2-(methylamino)-2-oxoethyl)-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate
| Conditions | Yield |
|---|---|
|
With N-ethyl-N,N-diisopropylamine; potassium iodide; In 1,4-dioxane; at 103 ℃; for 48h;
|
|
|
With N-ethyl-N,N-diisopropylamine; potassium iodide; In 1,4-dioxane; Reflux;
|
|
|
With N-ethyl-N,N-diisopropylamine; potassium iodide; In N,N-dimethyl acetamide; at 20 - 102 ℃; Solvent; Temperature;
|
-
![[4aS,9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole](/upload/2023/9/463380f0-97f4-4a9a-ae24-233496af6711.png)
- 1059630-07-7
[4aS,9bR]-6-bromo-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole

-
![(4aS,9bR)-ethyl 6-bromo-5-(2-(methylamino)-2-oxoethyl)-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/upload/2023/9/539ac3a6-91d2-449e-810f-3c9050eae495.png)
- 2098497-32-4
(4aS,9bR)-ethyl 6-bromo-5-(2-(methylamino)-2-oxoethyl)-1,3,4,4a,5,9b-hexahydro-2H-pyrido[4,3-b]indole-2-carboxylate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: triethylamine / tetrahydrofuran
2: potassium iodide; N-ethyl-N,N-diisopropylamine / 1,4-dioxane / Reflux
With triethylamine; N-ethyl-N,N-diisopropylamine; potassium iodide; In tetrahydrofuran; 1,4-dioxane;
|
|
|
Multi-step reaction with 2 steps
1: triethylamine / tetrahydrofuran / 2 h / 20 °C / Cooling with ice
2: potassium iodide; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 20 - 102 °C
With triethylamine; N-ethyl-N,N-diisopropylamine; potassium iodide; In tetrahydrofuran; N,N-dimethyl acetamide;
|
2098497-32-4 Upstream products
-
96-30-0

chloroacetylmethylamide
-
1059630-08-8
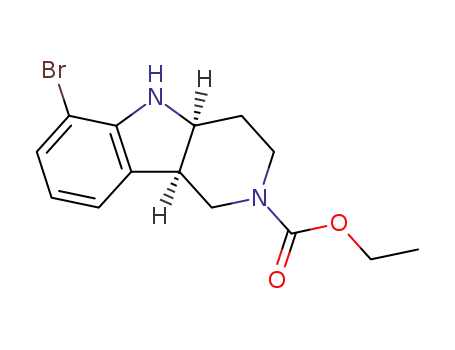
(4aS,9bR)-ethyl 6-bromo-3,4,4a,5-tetrahydro-1H-pyrido[4,3-b]indole-2(9bH)-carboxylate
-
50709-33-6
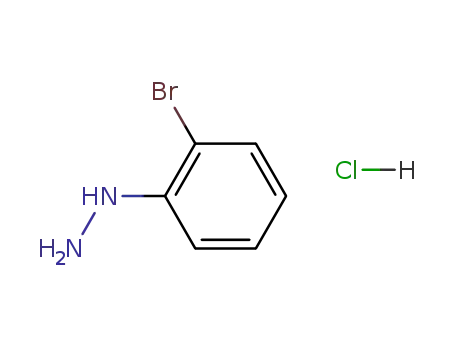
2-bromophenylhydrazine hydrochloride
-
40064-34-4
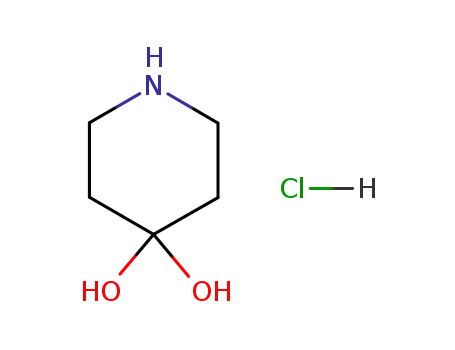
piperidine-4,4-diol hydrochloride
2098497-32-4 Downstream products
-
313369-25-4
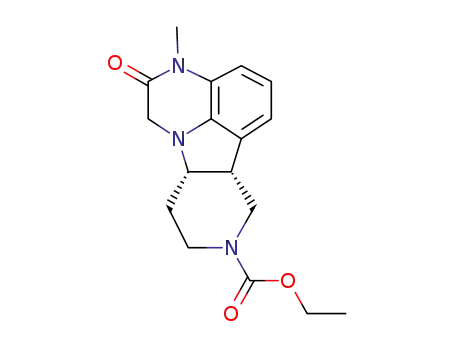
(6bR,10aS)-ethyl 2,3,6b,9,10,10a-hexahydro-3-methyl-2-oxo-1H-pyrido[3‘,4’:4,5]-pyrrolo[1,2,3-de]quinoxaline-8-carboxylate
-
313369-26-5
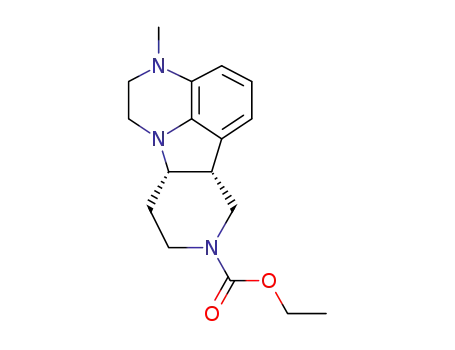
(6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3‘,4’:4,5]-pyrrolo[1,2,3-de]quinoxaline-8-carboxylate
-
313368-85-3
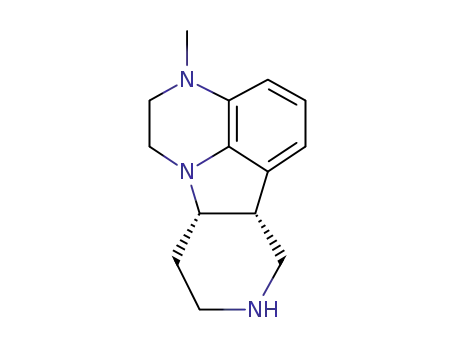
(6bR,10aS)-3-methyl-2,3,6b,7,8,9,10,10a-octahydro-1H-pyrido-[3’,4’:4,5]-pyrrolo[1,2,3-de]quinoxaline
相关产品
-
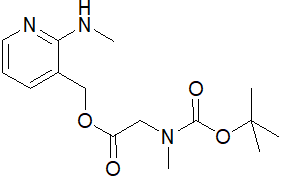
(2-(甲基氨基)吡啶-3-基)甲基 2-((叔丁氧羰基)(甲基)氨基)乙酸酯
CAS:1180002-01-0
-
(4aS,9bR)-6-溴-3,4,4a,5-四氢-1H-吡啶并[4,3-b]吲哚-2(9bH)-羧酸乙酯
CAS:1059630-08-8
-
爱德万甜一水合物
CAS:714229-20-6