142217-81-0
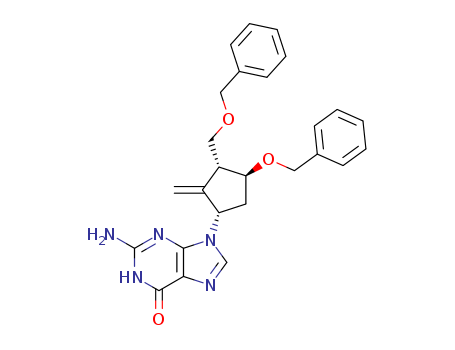
- Product Name:2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-(benzyloxy)-3-(benzyloxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one
- Structural Formula:C26H27N5O3
- Purity:99%
- Molecular Weight:457.532
Product Details
Factory supply commercial production 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-(benzyloxy)-3-(benzyloxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one 142217-81-0 manufacturer
- Molecular Formula:C26H27N5O3
- Molecular Weight:457.532
- Refractive Index:1.679
- PKA:9?+-.0.20(Predicted)
- PSA:108.05000
- Density:1.34 g/cm3
- LogP:4.20240
2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-(benzyloxy)-3-(benzyloxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one(Cas 142217-81-0) Usage
|
Chemical Properties |
White powder |
|
Uses |
3’5’Di-O-benzyl Entecavir is an intermediate in the preparation of the hepatitis B antiviral agent, Entecavir (E558900). |
InChI:InChI=1/C26H27N5O3/c1-17-20(15-33-13-18-8-4-2-5-9-18)22(34-14-19-10-6-3-7-11-19)12-21(17)31-16-28-23-24(31)29-26(27)30-25(23)32/h2-11,16,20-22H,1,12-15H2,(H3,27,29,30,32)/t20-,21-,22-/m0/s1
142217-81-0 Relevant articles
Improved entecavir intermediate synthesis process and improved entecavir synthesis process
-
Paragraph 0017; 0053; 0055; 0064; 0065; 0070, (2020/10/14)
The invention discloses an improved ente...
Entecavir preparation method using Boc protection group
-
Paragraph 0044; 0045; 0046, (2018/07/10)
The invention belongs to the field of dr...
A New Route for the Synthesis of Entecavir
Wang, Shan-chun,Zhang, Xi-quan,Gu, Hong-mei,Zhu, Xue-yan,Guo, Ya-jun
, p. 568 - 574 (2017/12/12)
-
A method for preparation of a purine derivative and intermediates
-
, (2016/12/01)
The invention discloses a preparation me...
142217-81-0 Process route
-
-
C38H41N5O5

-
![2-amino-1,9-dihydro-9-[(1S,3R,4S)-2-methylene-4-(phenylmethoxy)-3-[(phenylmethoxy)methyl]cyclopentyl]-6H-purin-6-one](/upload/2023/9/b5d326fd-035e-43a0-a1e9-a6b897070a4b.png)
-
142217-81-0
2-amino-1,9-dihydro-9-[(1S,3R,4S)-2-methylene-4-(phenylmethoxy)-3-[(phenylmethoxy)methyl]cyclopentyl]-6H-purin-6-one
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
tetrahydrofuran; methanol; water;
at 50 ℃;
for 2.5h;
|
92% |
-
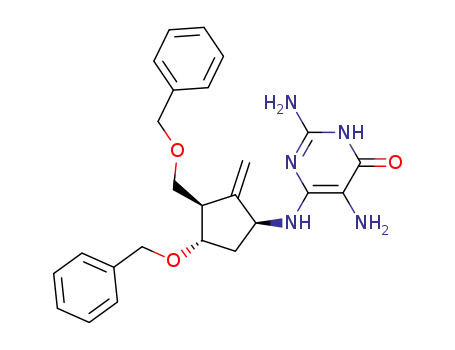
-
3,6-diamino-4-(((1S,3R,4S)-4-(benzyloxy)-3-((benzyloxy)methyl)-2-methylenecyclopentyl)amino)pyridin-2(1H)-one

-
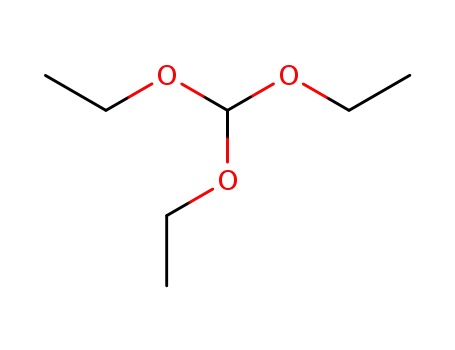
-
122-51-0
orthoformic acid triethyl ester

-
![2-amino-1,9-dihydro-9-[(1S,3R,4S)-2-methylene-4-(phenylmethoxy)-3-[(phenylmethoxy)methyl]cyclopentyl]-6H-purin-6-one](/upload/2023/9/b5d326fd-035e-43a0-a1e9-a6b897070a4b.png)
-
142217-81-0
2-amino-1,9-dihydro-9-[(1S,3R,4S)-2-methylene-4-(phenylmethoxy)-3-[(phenylmethoxy)methyl]cyclopentyl]-6H-purin-6-one
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol; water;
at 25 - 50 ℃;
for 12.16h;
Large scale;
|
53% |
142217-81-0 Upstream products
-
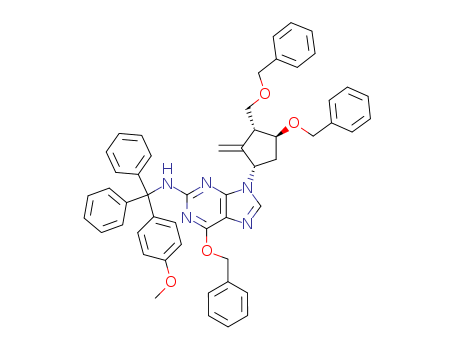
142217-80-9
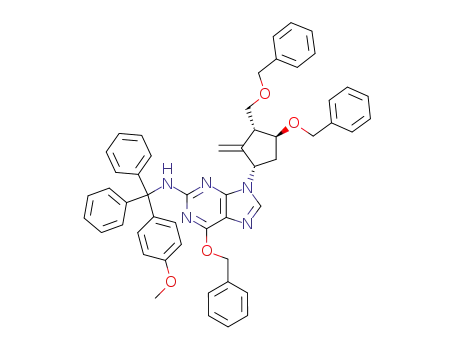
6-(benzyloxy)-9-((1S,3R,4S)-4-(benzyloxy)-3-((benzyloxy)methyl)-2-methylidenecyclopentyl)-N-((4-methoxyphenyl)diphenylmethyl)-9H-purin-2-amine
-
3587-60-8
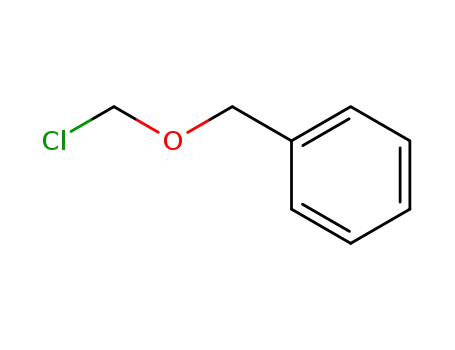
Benzyloxymethyl chloride
-
39939-07-6
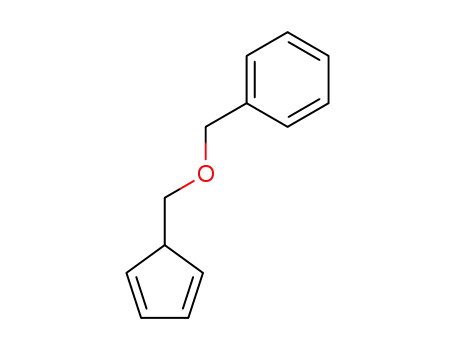
5-(benzyloxymethyl)cyclopentadiene
-
110567-22-1
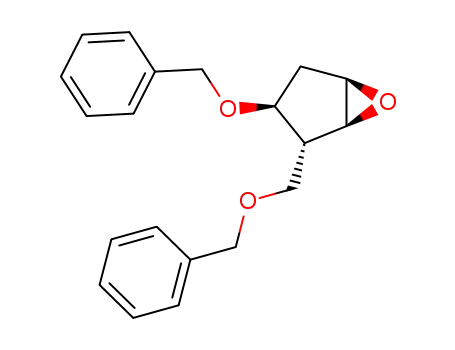
(2R,3S)-benzyloxy-2-benzyloxymethyl-6-oxabicyclo<3.1.0>hexane
142217-81-0 Downstream products
-
142217-69-4
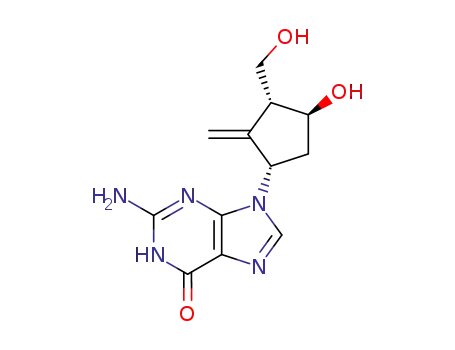
entecavir
Relevant Products
-
Exatecan mesylate; DX 8951f
CAS:169869-90-3
-
Pharmaceutical Intermediates intermediate
CAS:1286730-01-5