55094-52-5
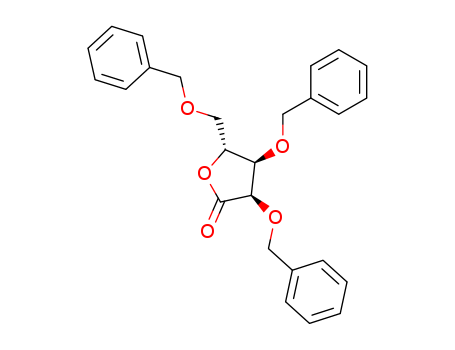
- Product Name:2,3,5-Tri-O-benzyl-D-ribonolactone
- Structural Formula:C26H26O5
- Purity:99%
- Molecular Weight:418.489
Product Details
pd_meltingpoint:54-55 °C
Factory supply Pharmaceutical Grade 2,3,5-Tri-O-benzyl-D-ribonolactone 55094-52-5 manufacturer
- Molecular Formula:C26H26O5
- Molecular Weight:418.489
- Melting Point:54-55 °C
- Boiling Point:576.8±50.0 °C(Predicted)
- PSA:53.99000
- Density:1.21±0.1 g/cm3(Predicted)
- LogP:4.29940
2,3,5-Tri-O-benzyl-D-ribonolactone(Cas 55094-52-5) Usage
|
Physical Form |
Solid |
|
Application |
2,3,5-Tri-O-benzyl-D-ribono-1,4-lactone is a useful research chemical. |
55094-52-5 Relevant articles
Synthesis and evaluation of a collection of purine-like C-nucleosides as antikinetoplastid agents
Bouton, Jakob,Maes, Louis,Karalic, Izet,Caljon, Guy,Van Calenbergh, Serge
supporting information, (2021/01/06)
The kinetoplastid parasites Trypanosoma ...
Compound for treating viral infection and preparation method and application of compound
-
Paragraph 0149-0154, (2021/08/07)
The invention provides a preparation for...
Compound containing guanidyl group, and preparation method and application thereof
-
Paragraph 0138-0143, (2021/08/07)
The invention provides a preparation con...
Novel compound and application thereof
-
Paragraph 0081-0083, (2021/09/08)
The invention relates to a novel compoun...
55094-52-5 Process route
-
-
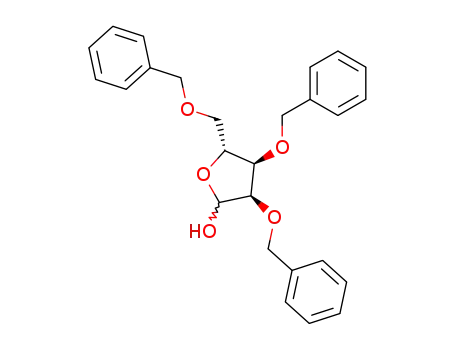
16838-89-4
2,3,5-tri-O-benzyl-D-ribofuranose

-
-
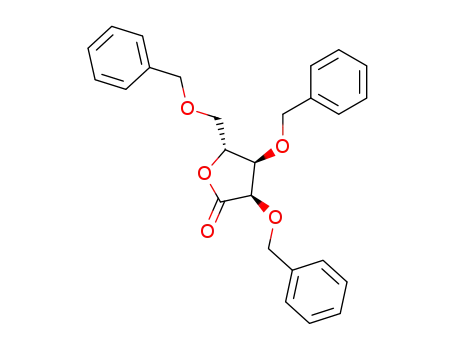
14233-64-8,134307-32-7,55094-52-5
(3R,4R,5R)-3,4-Bis-benzyloxy-5-benzyloxymethyl-dihydro-furan-2-one
| Conditions | Yield |
|---|---|
|
With
sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium thiosulfate; potassium bromide;
In
dichloromethane; water;
at 10 - 15 ℃;
for 0.00416667h;
under 1125.11 - 2250.23 Torr;
Temperature;
|
97.2% |
|
With
acetic anhydride; dimethyl sulfoxide;
at 20 ℃;
for 48h;
Inert atmosphere;
|
96% |
|
With
acetic anhydride; dimethyl sulfoxide;
at 20 ℃;
for 48h;
Inert atmosphere;
|
96% |
|
With
acetic anhydride; dimethyl sulfoxide;
at 20 ℃;
for 48h;
Inert atmosphere;
|
96% |
|
With
acetic anhydride; dimethyl sulfoxide;
at 20 ℃;
for 48h;
Reagent/catalyst;
Temperature;
Inert atmosphere;
|
96% |
|
With
acetic anhydride;
In
dimethyl sulfoxide;
at 20 ℃;
|
96% |
|
With
molecular sieve; tetrapropylammonium perruthennate; 4-methylmorpholine N-oxide;
In
dichloromethane;
at 20 ℃;
for 0.5h;
|
95% |
|
With
sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium bromide;
In
dichloromethane; water;
at 0 - 5 ℃;
for 2h;
Reagent/catalyst;
Temperature;
Large scale;
Green chemistry;
|
94.5% |
|
With
acetic anhydride;
In
dimethyl sulfoxide;
at 20 ℃;
for 48h;
Inert atmosphere;
|
93% |
|
With
acetic anhydride;
In
dimethyl sulfoxide;
at 20 ℃;
for 48h;
Inert atmosphere;
|
93% |
|
With
acetic anhydride;
In
dimethyl sulfoxide;
at 20 ℃;
for 48h;
|
93% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 20 ℃;
Molecular sieve;
Inert atmosphere;
|
90% |
|
With
acetic anhydride; dimethyl sulfoxide;
at 0 ℃;
|
89% |
|
With
acetic anhydride; dimethyl sulfoxide;
at 20 ℃;
for 12h;
|
86% |
|
With
sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium bromide;
at 0 ℃;
for 1h;
Temperature;
|
80.1% |
|
With
pyridinium chlorochromate;
In
dichloromethane;
at 40 ℃;
for 12h;
|
77.04% |
|
With
dipyridinium dichromate;
|
50% |
|
With
4 A molecular sieve; pyridinium chlorochromate;
Yield given;
|
|
|
Multi-step reaction with 4 steps
1: NaOEt; NH2OH*HCl / ethanol / 2.5 h / 55 °C
2: 92 percent / MnO2 / methanol / 14 h / Heating
3: 90 percent / Et3N / CH2Cl2 / 1 h / 30 °C
4: 44 percent / methanol / 1 h / 20 °C
With
manganese(IV) oxide; hydroxylamine hydrochloride; sodium ethanolate; triethylamine;
In
methanol; ethanol; dichloromethane;
|
|
|
2,3,5-tri-O-benzyl-D-ribofuranose;
With
oxalyl dichloride; dimethyl sulfoxide;
In
dichloromethane;
at -60 - -50 ℃;
for 1h;
With
triethylamine;
In
dichloromethane;
at -50 - 20 ℃;
for 1.16667h;
|
|
|
With
acetic anhydride; dimethyl sulfoxide;
at 20 ℃;
for 48h;
Reagent/catalyst;
Temperature;
Inert atmosphere;
|
|
|
With
oxalyl dichloride; dimethyl sulfoxide; triethylamine;
In
dichloromethane;
at -78 ℃;
|
|
|
With
sodium hypochlorite; dipotassium hydrogenphosphate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide;
In
tert-butyl methyl ether; water;
at 1 ℃;
|
|
|
With
sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; sodium hydrogencarbonate;
In
dichloromethane; water;
at 20 ℃;
|
180 g |
|
With
acetic anhydride;
In
dimethyl sulfoxide;
|
-
-
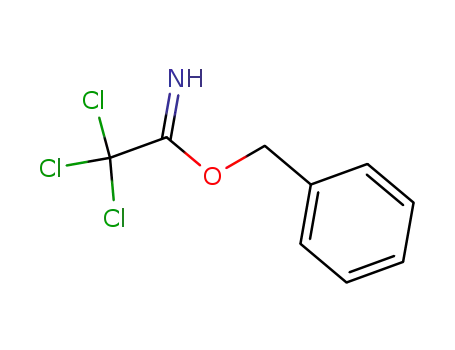
81927-55-1
O-benzyl 2,2,2-trichloroacetimidate

-
-
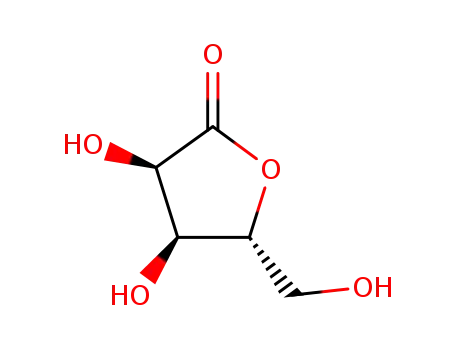
5336-08-3
D-Ribono-1,4-lactone

-

-
14233-64-8,134307-32-7,55094-52-5
(3R,4R,5R)-3,4-Bis-benzyloxy-5-benzyloxymethyl-dihydro-furan-2-one
| Conditions | Yield |
|---|---|
|
With
trifluorormethanesulfonic acid;
In
1,4-dioxane;
at 0 ℃;
for 3h;
Inert atmosphere;
|
80% |
|
With
trifluorormethanesulfonic acid;
In
1,4-dioxane;
pH 3;
|
70% |
55094-52-5 Upstream products
-
39085-59-1
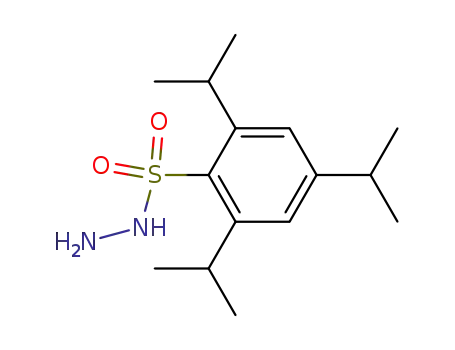
trisylhydrazine
-
89361-52-4
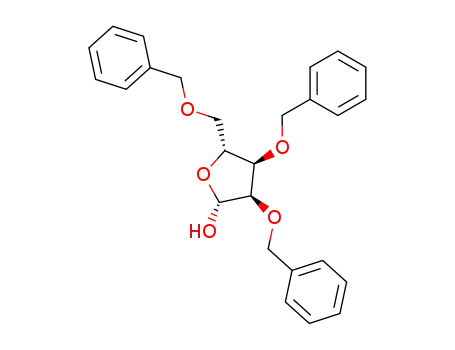
2,3,5-tris-O-(phenylmethyl)-β-D-ribofuranose
-
16838-89-4

2,3,5-tri-O-benzyl-D-ribofuranose
-
81927-55-1

O-benzyl 2,2,2-trichloroacetimidate
55094-52-5 Downstream products
-
160701-88-2
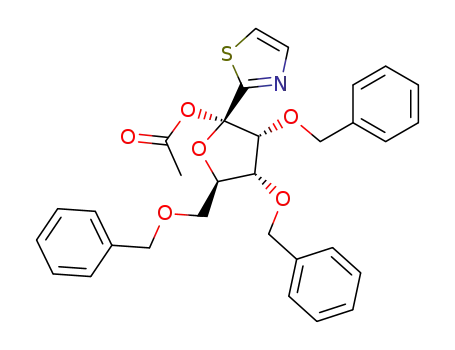
1-O-Acetyl-2,3,5-tri-O-benzyl-1-(2-thiazolyl)-α-D-ribofuranose
-
181582-02-5
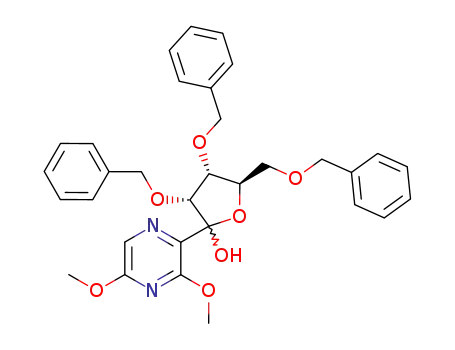
(3R,4R,5R)-3,4-Bis-benzyloxy-5-benzyloxymethyl-2-(3,5-dimethoxy-pyrazin-2-yl)-tetrahydro-furan-2-ol
-
181581-98-6
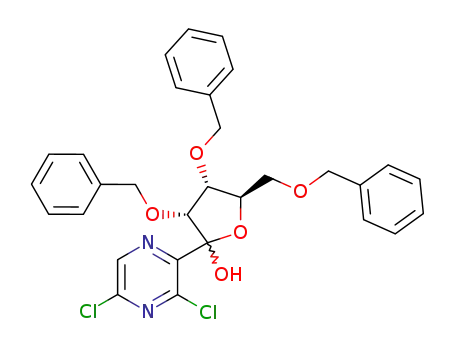
(3R,4R,5R)-3,4-Bis-benzyloxy-5-benzyloxymethyl-2-(3,5-dichloro-pyrazin-2-yl)-tetrahydro-furan-2-ol
-
181582-00-3
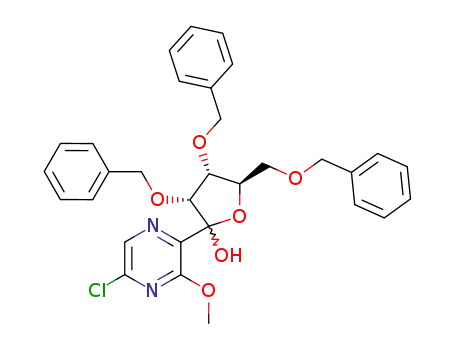
(3R,4R,5R)-3,4-Bis-benzyloxy-5-benzyloxymethyl-2-(5-chloro-3-methoxy-pyrazin-2-yl)-tetrahydro-furan-2-ol
Relevant Products
-
6-O-Benzylguanine
CAS:19916-73-5