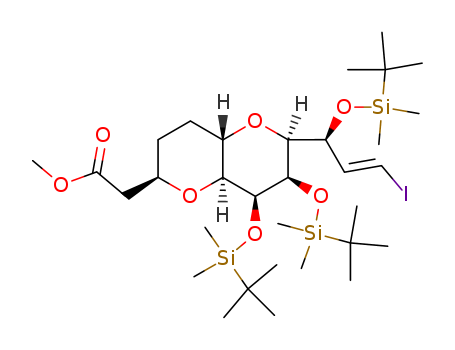
157322-47-9
- Product Name:[2S-[2alpha,5beta(3S*,5S*)]]-2,2-Dimethylpropanoic acid 3-[tetrahydro-5-(3-hydroxy-6-iodo-5-methyl-6-heptenyl)-4-methylene-2-furanyl]propyl ester
- Structural Formula:C21H35IO4
- Purity:99%
- Molecular Weight:478.411
Product Details
Factory Supply commercial production Fragment B Alcohol 157322-47-9 commercial production Manufacturer
- Molecular Formula:C21H35IO4
- Molecular Weight:478.411
- Boiling Point:491.8±45.0 °C(Predicted)
- PKA:14.89±0.20(Predicted)
- PSA:55.76000
- Density:1.27±0.1 g/cm3(Predicted)
- LogP:5.18560
157322-47-9 Relevant articles
Preparation method of eribulin intermediate
-
Paragraph 0085; 0086; 0087, (2020/11/09)
The invention provides a preparation met...
Compounds and synthesis method for Eribulin-B synthesis
-
Paragraph 0132-0158, (2020/05/02)
The invention relates to synthesis of an...
PROCESS FOR THE PREPARATION OF MACROCYCLIC KETONE ANALOGS OF HALICHONDRIN B
-
, (2019/11/19)
The present invention discloses a novel ...
PROCESS FOR PREPARATION OF ERIBULIN AND INTERMEDIATES THEREOF
-
, (2017/05/02)
The present application provides process...
157322-47-9 Process route
-
-
914922-88-6
C17H35NO3Si

-
-
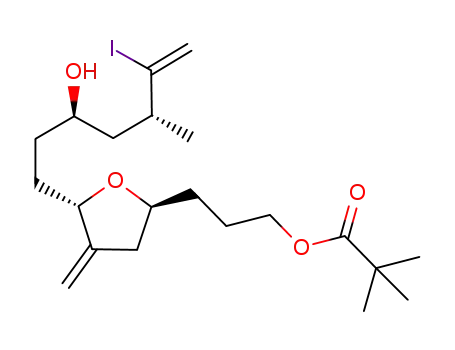
157322-47-9
3-((2S,5S)-5-((3R,5R)-3-hydroxy-6-iodo-5-methylhept-6-en-1-yl)-4-methylenetetrahydrofuran-2-yl)propyl pivalate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 9 steps
1.1: osmium(VIII) oxide; 4-methylmorpholine N-oxide / tetrahydrofuran; water; tert-butyl alcohol / 0 °C / Inert atmosphere
2.1: sodium periodate / tetrahydrofuran; water; tert-butyl alcohol / 6 h / 0 - 20 °C
3.1: chromium dichloride; triethylamine; nickel dichloride / tetrahydrofuran / 16 h / 0 - 20 °C / Inert atmosphere; Glovebox
4.1: silver trifluoromethanesulfonate; silver(II) oxide
5.1: tetrahydrofuran / 1 h / -20 - 20 °C / Inert atmosphere
6.1: triethylamine; hydrazine hydrate / ethanol / 2.5 h / 60 - 65 °C
7.1: triethylamine; iodine / diethyl ether / 0 - 20 °C
8.1: hydrogenchloride / methanol; isopropyl alcohol / 3 h / 0 - 20 °C / Inert atmosphere
9.1: 2,4,6-trimethyl-pyridine; dmap / 0.5 h / 0 °C / Inert atmosphere
9.2: 0.75 h / 20 °C / Inert atmosphere
With
2,4,6-trimethyl-pyridine; chromium dichloride; hydrogenchloride; dmap; silver(II) oxide; sodium periodate; osmium(VIII) oxide; hydrazine hydrate; iodine; silver trifluoromethanesulfonate; 4-methylmorpholine N-oxide; triethylamine; nickel dichloride;
In
tetrahydrofuran; methanol; diethyl ether; ethanol; water; isopropyl alcohol; tert-butyl alcohol;
|
-
-
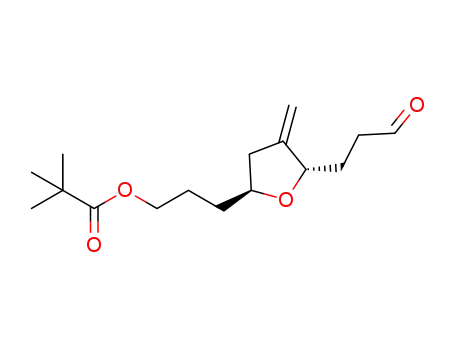
1443455-05-7
3-((2S,5S)-4-methylene-5-(3-oxopropyl)tetrahydrofuran-2-yl)propyl pivalate

-
-
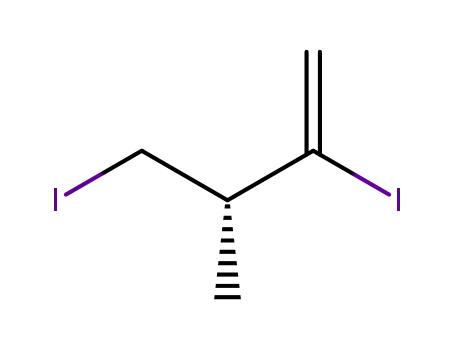
481048-22-0
(3R)-2,4-diiodo-3-methylbut-1-ene

-

-
157322-47-9
3-((2S,5S)-5-((3R,5R)-3-hydroxy-6-iodo-5-methylhept-6-en-1-yl)-4-methylenetetrahydrofuran-2-yl)propyl pivalate
| Conditions | Yield |
|---|---|
|
With
chromium chloride; zirconocene dichloride; cobalt(II) phthalocyanine; N-(2-((S)-4-isopropyl-4,5-dihydrooxazol-2-yl)-6-(((1S,2R,5S)-2-isopropyl-5-methylcyclohexyl)oxy)phenyl)benzenesulfonamide; magnesium; lithium chloride;
In
1,2-dimethoxyethane;
at 20 - 25 ℃;
for 31h;
Reagent/catalyst;
Glovebox;
|
92% |
|
With
2,6-dimethylpyridine; chromium dichloride; manganese; zirconocene dichloride; cobalt(II) phthalocyanine; triethylamine; lithium chloride;
In
tetrahydrofuran;
at 0 - 35 ℃;
for 18h;
Inert atmosphere;
Large scale;
|
55.9% |
157322-47-9 Upstream products
-
1159006-38-8
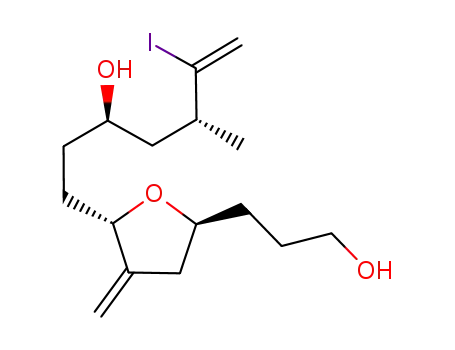
(3R,5R)-1-((2S,5S)-5-(3-hydroxypropyl)-3-methylenetetrahydrofuran-2-yl)-6-iodo-5-methylhept-6-en-3-ol
-
3282-30-2
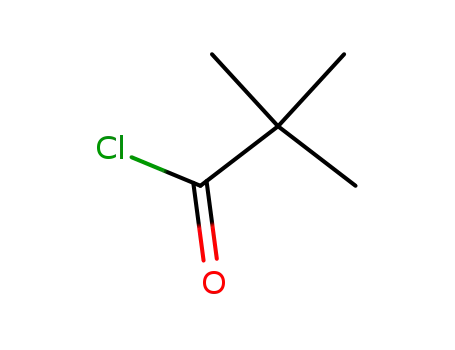
pivaloyl chloride
-
197229-73-5
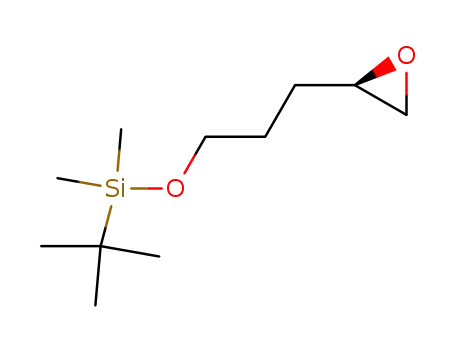
(2R)-2-[3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]propyl]oxirane
157322-47-9 Downstream products
-
253128-10-8
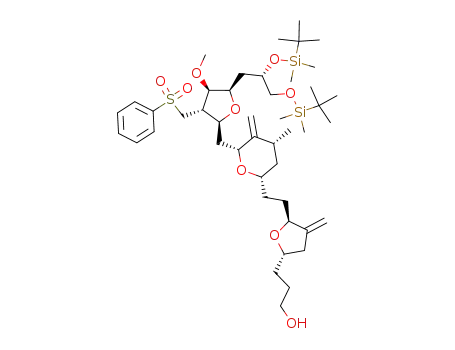
3-((2S,5S)-5-(2-((2S,4R,6R)-6-(((2S,3S,4R,5R)-5-((S)-2,3-bis((tert-butyldimethylsilyl)oxy)propyl)-4-methoxy-3-((phenylsulfonyl)methyl)tetrahydrofuran-2-yl)methyl)-4-methyl-5-methylenetetrahydro-2H-pyran-2-yl)ethyl)-4-methylenetetrahydrofuran-2-yl)propan-1-ol
-
480444-16-4
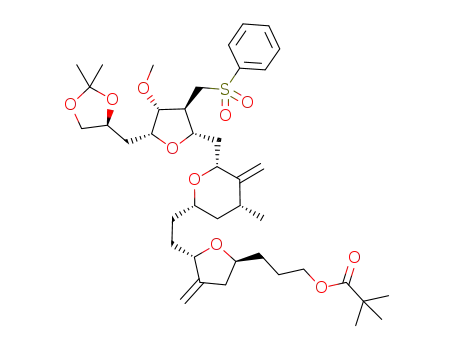
3-((2S,5S)-5-(2-((2S,4R,6R)-6-(((2S,3S,4R,5R)-5-(((S)-2,2-dimethyl-1,3-dioxolan-4-yl)methyl)-4-methoxy-3-((phenylsulfonyl)methyl)tetrahydrofuran-2-yl)methyl)-4-methyl-5-methylenetetrahydro-2H-pyran-2-yl)ethyl)-4-methylenetetrahydrofuran-2-yl)propyl pivalate
Relevant Products
-
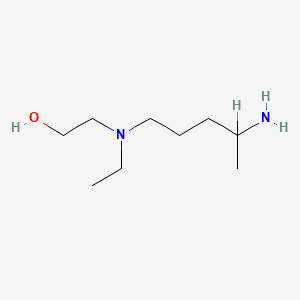
5-(N-Ethyl-N-2-hydroxyethylamino)-2-pentylamine
CAS:69559-11-1
-
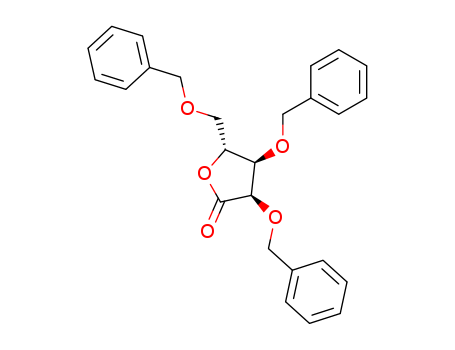
2,3,5-Tri-O-benzyl-D-ribonolactone
CAS:55094-52-5