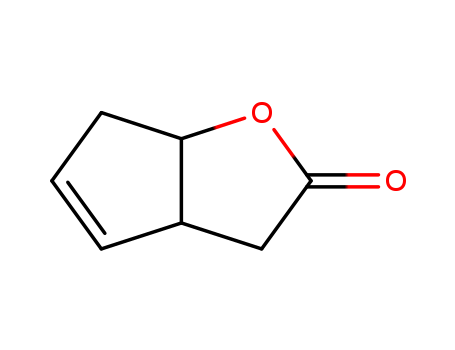
34638-25-0
- Product Name:3,3A,6,6A-TETRAHYDROCYCLOPENTA[B]FURAN-2-ONE
- Structural Formula:C7H8O2
- Purity:99%
- Molecular Weight:124.139
Product Details
factory supply Good Producer 3,3A,6,6A-TETRAHYDROCYCLOPENTA[B]FURAN-2-ONE 34638-25-0 manufacturer
- Molecular Formula:C7H8O2
- Molecular Weight:124.139
- Vapor Pressure:0.01mmHg at 25°C
- Boiling Point:263.139oC at 760 mmHg
- Flash Point:104.019oC
- PSA:26.30000
- Density:1.196g/cm3
- LogP:0.87800
3,3A,6,6A-TETRAHYDROCYCLOPENTA[B]FURAN-2-ONE(Cas 34638-25-0) Usage
InChI:InChI=1/C7H8O2/c8-7-4-5-2-1-3-6(5)9-7/h1-2,5-6H,3-4H2/t5-,6-/m1/s1
34638-25-0 Relevant articles
Towards practical baeyer-villiger-monooxygenases: Design of cyclohexanone monooxygenase mutants with enhanced oxidative stability
Opperman, Diederik J.,Reetz, Manfred T.
, p. 2589 - 2596 (2010)
Baeyer-Villiger monooxygenases (BVMOs) c...
Kinetic resolution of racemic 2-substituted 3-cyclopenten-1-ols by lipase-catalyzed transesterifications: A rational strategy to improve enantioselectivity
Ema, Tadashi,Maeno, Soichi,Takaya, Yusuke,Sakai, Takashi,Utaka, Masanori
, p. 8610 - 8616 (1996)
The effect of the acyl group of acylatin...
Lewis acidic Sn(IV) centers - Grafted onto MCM-41 - As catalytic sites for the Baeyer-Villiger oxidation with hydrogen peroxide
Corma, Avelino,Navarro, Maria Teresa,Renz, Michael
, p. 242 - 246 (2003)
Sn(IV) centers have been grafted onto me...
Aerobic Baeyer–Villiger Oxidation Catalyzed by a Flavin-Containing Enzyme Mimic in Water
Chevalier, Yoan,Lock Toy Ki, Yvette,le Nouen, Didier,Mahy, Jean-Pierre,Goddard, Jean-Philippe,Avenier, Frédéric
, p. 16412 - 16415 (2018)
Direct incorporation of molecular oxygen...
Preparation method corey lactone diol
-
, (2021/10/11)
The invention provides a preparation met...
Divorce in the two-component BVMO family: The single oxygenase for enantioselective chemo-enzymatic Baeyer-Villiger oxidations
R?llig, Robert,Paul, Caroline E.,Claeys-Bruno, Magalie,Duquesne, Katia,Kara, Selin,Alphand, Véronique
supporting information, p. 3441 - 3450 (2021/05/03)
Two-component flavoprotein monooxygenase...
Genome mining reveals new bacterial type I Baeyer-Villiger monooxygenases with (bio)synthetic potential
Bianchi, Dario A.,Carabajal, María Ayelén,Ceccoli, Romina D.,Rial, Daniela V.
, (2020/03/19)
Baeyer-Villiger monooxygenases (BVMOs) a...
Genome Mining of Oxidation Modules in trans-Acyltransferase Polyketide Synthases Reveals a Culturable Source for Lobatamides
Ueoka, Reiko,Meoded, Roy A.,Gran-Scheuch, Alejandro,Bhushan, Agneya,Fraaije, Marco W.,Piel, J?rn
supporting information, p. 7761 - 7765 (2020/03/25)
Bacterial trans-acyltransferase polyketi...
34638-25-0 Process route
-
![bicyclo[3.2.0]hept-2-en-6-one](/upload/2023/9/6a6e55bf-a18a-4355-a814-14535847a27d.png)
-
13173-09-6,62182-73-4,71155-04-9,71155-05-0
bicyclo[3.2.0]hept-2-en-6-one

-
![(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/f5eaac48-8df2-4d60-b518-1f6ad9041d24.png)
-
34638-25-0,38110-77-9,43119-28-4,54483-22-6,26054-46-6
(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
![(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/0a7db7d8-8441-4a1d-be1c-dc886ba2465e.png)
-
26054-46-6
(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
![3-oxabicyclo[3.3.0]oct-6-en-2-one](/upload/2023/9/a7645950-b698-4740-a220-6bbcfc41d6ed.png)
-
128946-78-1
3-oxabicyclo[3.3.0]oct-6-en-2-one

-
-
103618-27-5
(+)-(1S,5R)-3-oxabicyclo<3.3.0>oct-6-en-2-one
| Conditions | Yield |
|---|---|
|
With
oxygen;
In
water;
at 30 ℃;
for 2h;
Yield given. Yields of byproduct given;
biotransformation by Ps. putida NCIMB 10007 monooxygenase enzyme using NADPH as cofactor; further monooxygenases; pH 7.1;
|
|
|
With
Cumene hydroperoxide; (S)-[1,1']-binaphthalenyl-2,2'-diol; dimethylaluminum chloride;
In
hexane; toluene;
at -25 - 20 ℃;
for 12h;
Title compound not separated from byproducts;
|
|
|
With
2CF3O3S(1-)*C56H46O2P2Pt(2+); dihydrogen peroxide;
In
water;
at 5 ℃;
for 5h;
optical yield given as %ee;
enantioselective reaction;
|
|
|
With
secondary alcohol dehydrogenase; wild-type phenylacetone monooxygenase; NADP; isopropyl alcohol;
In
acetonitrile;
at 30 ℃;
pH=8;
optical yield given as %ee;
enantioselective reaction;
aq. buffer;
Enzymatic reaction;
|
|
|
With
C52H29O4P; dihydrogen peroxide;
In
dichloromethane; water;
at -40 ℃;
for 36h;
enantioselective reaction;
|
-
![bicyclo[3.2.0]hept-2-en-6-one](/upload/2023/9/6a6e55bf-a18a-4355-a814-14535847a27d.png)
-
13173-09-6,62182-73-4,71155-04-9,71155-05-0
bicyclo[3.2.0]hept-2-en-6-one

-
![(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/f5eaac48-8df2-4d60-b518-1f6ad9041d24.png)
-
34638-25-0,38110-77-9,43119-28-4,54483-22-6,26054-46-6
(1R,5S)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
![(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one](/upload/2023/9/0a7db7d8-8441-4a1d-be1c-dc886ba2465e.png)
-
26054-46-6
(-)-(1S,5R)-2-oxabicyclo[3.3.0]oct-6-en-3-one

-
-
103618-27-5
(+)-(1S,5R)-3-oxabicyclo<3.3.0>oct-6-en-2-one
| Conditions | Yield |
|---|---|
|
With
oxygen;
In
water;
at 30 ℃;
for 1h;
Yield given. Title compound not separated from byproducts;
biotransformation by Ps. putida NCIMB 10007 monooxygenase enzyme using NADH as cofactor; further monooxygenases; pH 7.1;
|
47 % Chromat. |
|
With
oxygen;
In
water;
at 30 ℃;
for 1h;
Yields of byproduct given;
biotransformation by Ps. putida NCIMB 10007 monooxygenase enzyme using NADH as cofactor; further monooxygenases; pH 7.1;
|
47 % Chromat. |
|
With
phenylacetone monooxygenase Pro440Trp mutant; secondary alcohol dehydrogenase; NADP; isopropyl alcohol;
In
acetonitrile;
at 30 ℃;
pH=8;
optical yield given as %ee;
enantioselective reaction;
aq. buffer;
Enzymatic reaction;
|
|
|
With
D-glucose;
In
water; N,N-dimethyl-formamide;
at 20 ℃;
for 48h;
Reagent/catalyst;
regioselective reaction;
Enzymatic reaction;
|
34638-25-0 Upstream products
-
925211-06-9
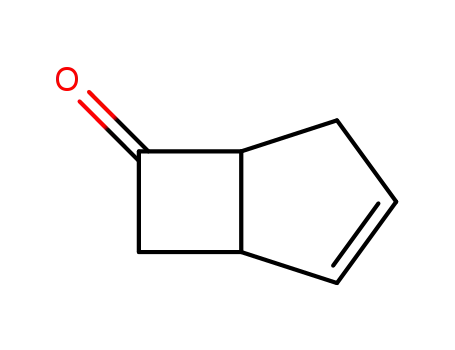
bicyclo(3.2.0)hept-2-en-6-one
-
124938-45-0
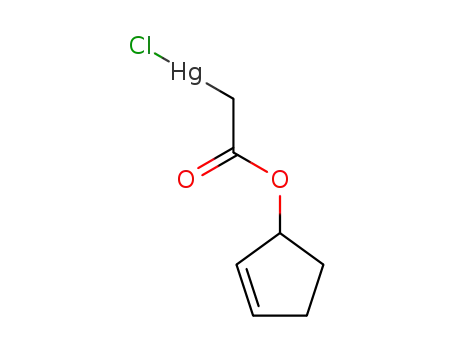
C7H9ClHgO2
-
3234-54-6
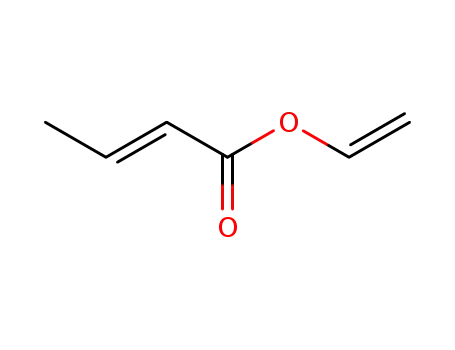
vinyl crotonate
-
606490-73-7
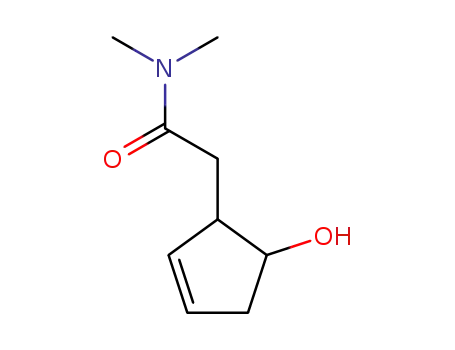
2-(5-Hydroxy-cyclopent-2-enyl)-N,N-dimethyl-acetamide
34638-25-0 Downstream products
-
88420-92-2
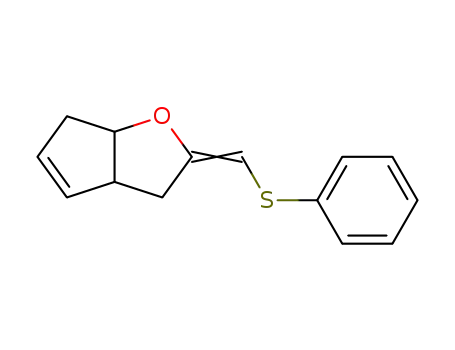
2-[1-Phenylsulfanyl-meth-(E)-ylidene]-3,3a,6,6a-tetrahydro-2H-cyclopenta[b]furan
-
434313-66-3
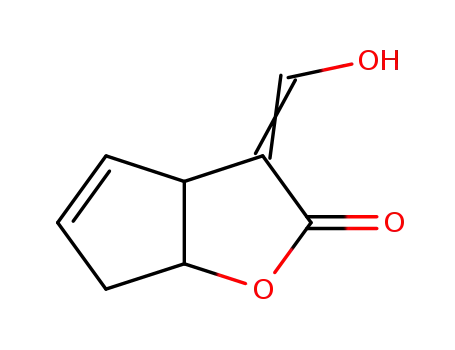
3-hydroxymethylene-3,3a,6,6a-tetrahydrocyclopentafuran-2-one
-
78646-91-0
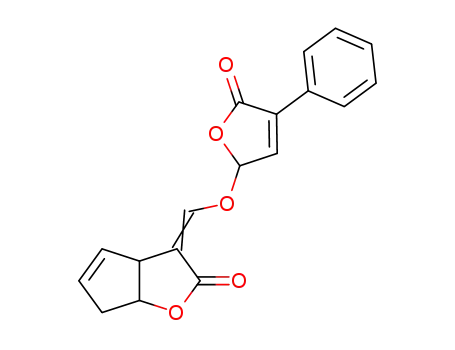
4-(2-oxo-3,3a,6,6a-tetrahydro-2H-cyclopentafuran-3-ylidenemethoxy)-2-phenylbut-2-en-4-olide
-
78646-92-1
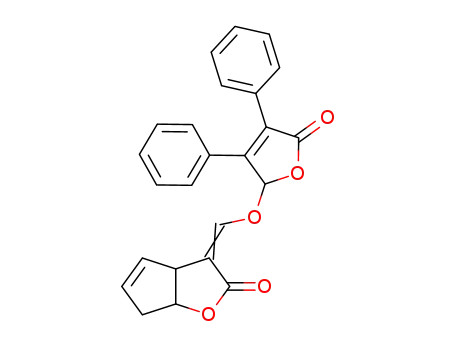
4-(2-oxo-3,3a,6,6a-tetrahydro-2H-cyclopentafuran-3-ylidenemethoxy)-2,3-diphenylbut-2-en-4-olide
Relevant Products
-
Pharmaceutical Intermediates intermediate
CAS:1286730-01-5